When I was doing research for Body Hack IV with Bill Andrews and the studies trying to stop senescence and increase lifespan, I came across a substance that many medical professional shave started to say has a really powerful anti-aging effect. The substance is L-Carnosine.
From the website JonBarron.Com
End Of Old Age
I’m not a big believer in magic bullets. Everything I’ve ever learned says that you’re only as strong as your weakest link. That’s why I’ve always preached that the key to health is raising your entire Baseline of Health. But that said, I have to admit that what we’re talking about here today is a uniquely important anti-aging discovery.
What Is Aging?
The best place to start is at the beginning. What is aging? What makes us age? There are actually many factors that contribute to old age (free radical damage, hormonal changes, etc.), but of all of the things that make us “old,” two things stand out because until now, they have been so untouchable:
- The Hayflick Limit
- The glycation of proteins
The Hayflick Limit: Cell Life Span
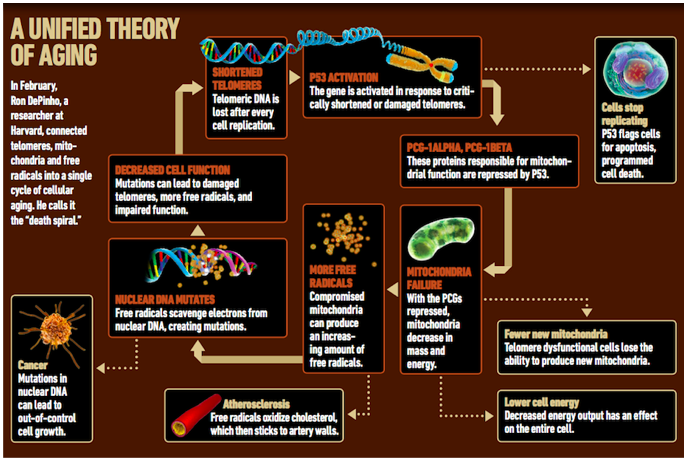
The Hayflick Limit is named after the person who discovered it almost 40 years ago. A quick description is that all cells have only a limited capacity to continue to divide through the course of our lives.
Those numbers are different for each type of cell in our body, and by early adulthood, half of those divisions have been used up. By mid-life, maybe only 20-39% of those divisions are left. At that point, old age starts taking over – then death.
This limited capacity of a cell to perpetuate itself is called the Hayflick Limit. In effect, the Hayflick Limit determines life span at the cellular level. With each division, a cell becomes less likely to divide again, until finally it stops dividing altogether and becomes what we call senescent.
Cell senescence is the final step before cell death. Senescent cells are still alive and metabolically active, but they’re no longer capable of dividing. More importantly, though, senescent cells exhibit all of the characteristics that so bother us about old age, such as the difference between the supple skin of a child and the wrinkled skin of the elderly.
How do cells age?
As cells approach the Hayflick Limit, they divide less frequently and become aberrant. They take on wildly irregular forms. They no longer line up in parallel arrays; they assume a granular appearance, and deviate from their normal size and shape. This distorted appearance, called the senescent phenotype, is accompanied by a state of declining functionality that, UNTIL RECENTLY, was thought to be irreversible.
Astounding News: Reverse Aging
As it turns out, not only can we reverse the aging process at the cellular level now, and actually do it quite simply AND QUICKLY – but we can also reverse aging at the system level and the organ level. And for that matter, we can reverse it in terms of how we look and feel – and by that I mean our skin and hair and energy levels. And then, of course, we can even reverse aging in terms of lifespan.
What’s the Secret?
The substance I’m talking about is L-carnosine. It’s a naturally occurring combination of two amino acids, alanine and histidine, that was discovered in Russia in the early 1900s. Because much of the research was done in Russia, it has been largely unavailable in the United States until recently. Now, though, there have been a number of studies and experiments in other parts of the world verifying everything done in Russia – and more.
Most notably, there were a series of astonishing experiments done in Australia that proved that carnosine rejuvenates cells as they approach senescence. Cells cultured with carnosine lived longer and retained their youthful appearance and growth patterns.
What’s probably the most exciting result of the studies is that it was discovered that carnosine can actually REVERSE the signs of aging in senescent cells.
The Reversal of Aging
When the scientists transferred senescent cells to a culture medium containing carnosine, those cells exhibited a rejuvenated appearance and often an enhanced capacity to divide. When they transferred the cells back to a medium lacking carnosine, the signs of senescence quickly reappeared.
As they switched the cells back and forth several times between the culture media, they consistently observed that the carnosine medium restored the juvenile cell phenotype WITHIN DAYS, whereas the standard culture medium brought back the senescent cell phenotype.
Increase Cell Life
In addition, the carnosine medium increased cell life span — even for old cells. When the researchers took old cells that had already gone through 55 divisions and transferred them to the carnosine medium, they survived up to 70 divisions, compared to only 57 to 61 divisions for the cells that were not transferred.
This represents an increase in the number of cell divisions for each cell of almost 25%.
But in terms of cell life, the increase was an astounding 300%. The cells transferred to the carnosine medium attained a life span of 413 days, compared to just 126 to 139 days for the control cells.
Increase Life Expectancy
This is mind-boggling. But so far, all we’ve talked about are cells. What does carnosine mean for actual life expectancy?
A new Russian study on mice has shown that mice given carnosine are twice as likely to reach their maximum lifespan as untreated mice. The carnosine also significantly reduced the outward “signs of old age.”
In effect, it made the mice look younger. 44% of the carnosine treated mice had young, glossy coats in old age as opposed to only 5% in the untreated mice. This represents 900% better odds of looking young in old age.
Feel Young Again
Another important difference between the treated and the untreated mice was in their behavior. Only 9% of the untreated mice behaved youthfully in old age, versus 58% of the carnosine treated mice. That’s a 600% improvement in how they felt.
Strong Antioxidant
Quite simply, carnosine is one of the most powerful antioxidants known. It’s a great heavy-metal scavenger. It’s a powerful auto-regulator. And it stands alone when it comes to preventing and reversing protein glycation or cross-linking.
Auto-Regulator
Carnosine has the remarkable ability to throttle down bodily processes that are in a state of excess, and to ramp up those that are under expressed.
For example, carnosine thins the blood of people whose blood tends to clot too much andincreases the clotting tendency in those with a low clotting index.
Another example is that carnosine suppresses excess immune responses in those who have “hyper” immune systems, whereas it stimulates the immune response in those with weakened immune systems – such as the aged.
And carnosine even seems to have the ability to normalize brain wave functions.
Protein Glycation: Sugar And Aging
Glycation is the uncontrolled reaction of sugars with proteins. It’s kind of like what happens to sugars when you heat them and they caramelize. In effect, glycation is what happens when excess sugars caramelize the proteins in your body. It’s a major factor in the aging process – and it’s particularly devastating to diabetics.
Your body is mostly made up of proteins. In fact, proteins are the substances most responsible for the daily functioning of your body. That’s why anything that causes protein deterioration has such a dramatic impact on the body’s function and appearance.
Thanks largely to the destructive effect of sugar and aldehydes, the protein in our bodies tends to undergo destructive changes as we age. This destruction is a prime factor, not only in the aging process itself, but also in the familiar signs of aging such as wrinkling skin, cataracts, and the destruction of our nervous system – particularly our brains. Studies show that carnosine is effective against all these forms of protein modification.
Protein Modification for Longevity
As I said, aging is associated with damage to cellular proteins. But carnosine protects cellular proteins from damage in at least two ways.
- First, it bonds with the carbonyl (or aldehyde) groups that if left alone will attack and bind with proteins.
- Second, it works as an antioxidant to prevent the formation of oxidized sugars, also calledAdvanced Glycosylation End-products or AGEs for short. That’s really the caramelization thing that I mentioned earlier. The bottom line here is that the less AGEs, in your body, the younger you are.
Both of these processes have important implications for anti-aging therapy. The key is that carnosine not only prevents damaging cross-links from forming, it eliminates cross-links that have previously formed in proteins, thus restoring normal membrane function.
Alzheimer’s
Carnosine has been proven to reduce or completely prevent cell damage caused by beta amyloid, one of the prime protein risk factors for Alzheimers. The presence of beta amyloid leads to damage of the nerves and arteries of the brain. Carnosine blocks and inactivates beta amyloid. In effect, it protects neural tissues against dementia. The key is that carnosine not only prevents damaging cross-links from forming in proteins, it eliminates cross-links that have previously formed in those proteins, thus restoring normal membrane function in cells. This is true not only in the brain, but in all the organs of our body – our skin included. Keep in mind that the damage you see in the skin is not just a cosmetic question. That damage is absolutely an indicator of the kinds of damage happening to every other organ in your body – including your eyes and your brain.
The Reversal of Age
Carnosine levels in our body directly correlate with both the length and quality of our lives. And since carnosine levels decline with age, supplementation with carnosine represents one of the most powerful things you can do to hold back the ravages of old age.
Results
While it is true that many people who supplement with carnosine are going to notice everything from younger looking skin to more energy, the bottom line is that you really shouldn’t look for any short term benefits from carnosine supplementation. If any short-term benefits are noticed, you should consider them an added bonus.
The reason you want to supplement with carnosine is for the long term, not for the short-term benefits that you may or may not notice. You supplement with carnosine to protect against the long-term ravages of aging.
Using Carnosine
Some experts recommend using only 50-100 mg of carnosine a day. Others say that if you don’t take 1,000-1,500 mg a day it won’t work because your body metabolizes the first 500 mg or so.
The key here is that all of these experts are ignoring the simple fact, that different people need different amounts. For example:
- The older you get, the more you need.
- If you eat a mostly vegetarian diet, you need more.
- If you’re diabetic, or just have trouble with blood sugar, you need more.
I think most people will do best on 500-750 mg a day.
If you’re young and healthy and include meat in your diet, then 250 mg a day makes sense. As you get older, and if you’re starting to show signs of aging or glycation (such as cataracts), then you’d want to think of increasing the dosage up to 1,000 mg a day – maybe even as high as 1,500 mg a day.
Safety
In studies, carnosine has been proven safe in amounts as high as 70, 80, or even 100 grams a day, although a small number of people have noticed some minor muscle twitching at doses as small as 1,000 mg. The bottom line is use what you need, and you won’t have any problems – only benefits.
Importance
As I mentioned earlier, I don’t believe in magic bullets. Everything I’ve ever learned says that you’re only as strong as your weakest link. I still believe that improving your entire Baseline of Health® is the key to good health and long life.
But that said, I think that once you actually understand what carnosine does – once you understand the role it plays in preventing and potentially reversing all of the signs of old age in the body (and we’re talking about everything from wrinkled skin to cataracts to Alzheimer’s) – heck, once you understand the role it plays in extending life itself – then you’re left with the unmistakable conclusion that supplementing with carnosine may represent one of the single best things you can do to help “turn back your biological clock.”
A Missing Link
As important as carnosine is, there is a “gap” in its usefulness. It’s called lipofuscin.
Lipofuscin is the age pigment commonly found in aging brains and in other tissue such as the skin. By itself, it is not dangerous. It is merely a byproduct of harmful reactions that have already taken place. For example, one of the byproducts of free radical damage and protein/aldehyde damage (both conditions that carnosine addresses) is lipofuscin.

Lipofuscin deposits as seen in heart muscle
When you supplement with carnosine, however, something different happens. The carnosine quickly binds with the aldehydes, preventing them from damaging the proteins. The byproduct of this reaction is lipofuscin. So once again you have inactive lipofuscin compounds, but this time as the result of PREVENTING protein damage. In a sense, with carnosine you trade protein damage for lipofuscin.
As I said before, by itself, lipofuscin is not harmful. However, if enough of it accumulates over time (and this process is accelerated when you supplement with carnosine), it can interfere with proper cellular and organ functions. So the bottom line is that however it is produced (as a result of protein damage, or as the result of taking sacrificial carnosine to prevent protein damage), you want to get rid of it.
DMAE
By any definition, DMAE is the perfect companion to carnosine in an anti-aging formulation. First, it reinforces carnosine’s own anti-aging properties. Then, it provides a whole series of complementary benefits of its own.
What Is DMAE?
DMAE is short for (dimethylaminoethanol), a naturally-occurring nutrient that enhances acetylcholine (ACh) synthesis. Adequate levels of ACh are important for proper memory function. Normally found in small amounts in our brains, DMAE has been shown to remarkably enhance brain function when used as a supplement in clinical studies.
DMAE Reinforces Carnosine
One of the prime actions of DMAE is that it flushes accumulated lipofuscin from your body – from the neurons in your brain, from your skin, and from all other organs. It also complements carnosine in that DMAE on its own has been shown to inhibit and reverse the Cross-Linking of proteins and extend lifespan.
Many people have heard of the anti-aging results that Romanian scientist, Ana Aslan, achieved using something called GH3, or procaine. What most people do not know is that GH3 breaks down in the body to form DMAE (after first metabolizing into DEAE) and PABA. In other words, DMAE is the key active component in Ana Aslan’s anti-aging formula.
Numerous scientific studies now show that DMAE can help:
- Increase Acetylcholine levels and RNA levels in the brain
- Stimulate mental activity
- Increase attention span
- Increase alertness
- Increase intelligence (especially in children)
- Improve learning and memory
- Increase energy levels
- Provide a mild, safe tonic effect
- Stimulate the central nervous system
- Relieve anxiety
- Elevate mood in general
- Alleviate behavioral problems and hyperactivity associated with Attention Deficit Disorder
- Increase motivation and reduce apathy in persons suffering from depression
- Induce sounder sleep
- Over time reduce the amount of sleep required by about 1 hour per night
- Intensify dreams tremendously. (Even more so when you take it along with a large dose of phosphatidyl choline — a key component of lecithin)
- Cause dreams to become more lucid
- Increase willpower
- Decrease the incidence and severity of hangovers in people who consume excessive amounts of Alcohol
DMAE Is Safe
Clinical studies of DMAE have used up to 1,600 mg per day with no reports of side effects. In some cases, some people may experience slight headaches, muscle tension, or insomnia if they take too much too soon.
These effects are easily eliminated if intake is reduced and then gradually increased. Although there is no direct connection, many manufacturers recommend that women who are pregnant or breast-feeding, anyone who suffers from convulsions, epilepsy, or seizure disorders, and people with manic-depressive illness should avoid using DMAE.
This is probably more of a legal issue than a medical issue.
Acetyl-L-Carnitine
Like DMAE, acetyl-L-carnitine is a perfect complement to L-carnosine.
Although your body can synthesize L-carnitine in the liver, it depends on outside sources (meat being a primary source) to fulfill its requirements. This can present a problem for vegetarians since L-carnitine performs several key functions in the human body. For one, it can improve the functioning of the immune system by enhancing the ability of macrophages to function as phagocytes. And it can improve the functioning of muscle tissue. In fact, it has been shown to increase running speed when given prior to exercise. It also plays a major factor in cellular energy production by shuttling fatty acids from the main cell body into the mitochondria (the cell’s energy factories) so that the fats can be oxidized for energy. Without carnitine, fatty acids cannot easily enter the mitochondria.
There is, however, a specialized form of L-carnitine known as acetyl-L-carnitine (ALC) that is often deficient even in meat eaters and that performs virtually all of the same functions – but better. For example, in terms of cellular energy production, in addition to shuttling fatty acids into cell mitochondria, ALC provides acetyl groups from which Acetyl-Coenzyme A (a key metabolic intermediate) can be regenerated, thereby facilitating the transport of metabolic energy and boosting mitochondrial activity. But beyond that, the addition of the acetyl group makes ALC water soluble, which enables it not only to diffuse easily across the inner wall of the mitochondria but also to cross all cell membranes more easily. In other words, ALC reaches parts of the body where L-carnitine cannot go. In particular, ALC readily crosses the blood/brain barrier, where it provides a number of specialized neurological functions. For example, it can:
- Facilitate both the release and synthesis of acetylcholine, a key brain biochemical.
- Increase the brain’s levels of choline acetylase.
- Enhance the release of dopamine and improve the binding of dopamine to dopamine receptors.
- Protect the neurons of the optic nerve and the occipital cortex of the brain.
In addition, studies have shown that acetyl-L-carnitine can inhibit the deterioration in mental function associated with Alzheimer’s disease and slow its progression. Part of this is a result of its ability to shield neurons from the toxicity of beta amyloid protein. As a result:
- ALC improves alertness in Alzheimer’s patients.
- Improves attention span.
- And it increases short term memory.
Through its action on dopamine (a chemical messenger used between nerve cells) and dopamine receptors, ALC seems to play a major role in preventing and/or minimizing the symptoms of Parkinson’s disease.
- ALC enhances the release of dopamine from dopaminergic neurons and improves the binding of dopamine to dopamine Receptors.
- ALC retards the decline in the number of dopamine receptors that occurs as part of the normal aging process and (more rapidly) with the onset of Parkinson’s disease. In fact, many researchers believe that Parkinson’s may be caused by a deficiency of dopamine.
- And ALC inhibits tremors.
And acetyl-L-carnitine may even play a role in helping with MS.
- ALC inhibits (and possibly reverses) the degeneration of myelin sheaths
But most of all, ALC just helps slow down the aging process of the brain.
- ALC retards the inevitable decline in the number of glucocorticoid teceptors that occurs with aging.
- It retards the age-related deterioration of the hippocampus.
- It retards the inevitable decline in the number of nerve growth factor receptors that occurs as we age.
- It stimulates and maintains the growth of new neurons within the brain (both independently of Nerve Growth Factor (NGF) and as a result of preserving NGF) and helps to prevent the death of existing neurons.
- ALC protects the NMDA receptors in the brain from age-related decline.
- ALC inhibits the excessive release of adrenalin in response to stress and inhibits the depletion of luteinising hormone releasing hormone and testosterone that occurs as a result of excessive stress.
- And ALC enhances the function of cytochrome oxidase, an essential enzyme of the Electron Transport System.
The mind boosting effect of acetyl-L-carnitine is often noticed within a few hours — or even within an hour — of supplementing. Most people report feeling mentally sharper, having more focus, and being more alert. Some find a mild mood enhancement. More specifically:
- ALC improves learning ability along with both short term and long term memory
- It improves mood by 53%.
- It both improves the quality of and reduces the need for sleep.
- It improves verbal fluency.
- And ALC improves hand eye coordination by some 300-400%.
And yes, acetyl-L-carnitine helps flush lipofuscin from the body — especially from the brain.
The Longevity Bottom Line
Based on everything we know, supplementing with a combination of L-carnosine, DMAE, and acetyl-L-carnitine is one of the simplest, most effective, and safest steps we can take to help turn back the clock and optimize our health.
From the website emaxhealth.com I copy and pasted the article that talks about the possible benefits towards anti-aging with L-Carnosine.
|
L-Carnosine was recently touted on The Dr. Oz Show as a miracle anti-aging pill and has been linked to preventing telomere shortening. The latest research shows that telomere shortening is directly linked to heart attacks and premature death. Could L-Carnosine be the answer to anti-aging?
In a soon to be published article in the scientific journal Arteriosclerosis, Thrombosis and Vascular Biology published by the American Heart Association, researchers involved in cellular aging have discovered the striking finding that telomere shortening is directly linked to heart attacks and premature death. Telomeres are specialized repeating segments of DNA that cap the ends of chromosomes. The primary function of telomeres is to protect the free ends of chromosomes from losing base pair sequences at their ends and to prevent chromosomes from fusing to each other. During the natural life cycle of a cell, cell growth and aging involves a replication process called mitosis where a parent cell will double its amount of DNA and then split into two daughter cells, each with a normal amount of chromosomal DNA. During this process, telomeres protect the ends of the chromosomes from a gradual loss at their ends. A telomere is a repeating DNA sequence (TTAGGG for example) at the ends of chromosomes that can reach a length of several thousand base pairs. While the telomeres protect the ends of chromosomes from eroding away, each time a cell divides some of the telomere DNA sequence (approximately 25-200 base pairs at a time) is lost. The result is that telomeres gradually wear away. At some point in a cell’s life after many cell divisions, a telomere becomes too short and the cell can no longer replicate. The cell is then considered to be relatively old and then dies by a process called apoptosis—a normal process in cell aging. In the aforementioned study to be published by the American Heart Association, researchers from the University of Copenhagen conducted a large scale study involving almost 20,000 individuals during a time period of nearly 19 years. In the study, each individual’s DNA was isolated and analyzed to determine their specific telomere length. Their research was based on previous studies that showed that smoking and obesity cause telomeres to shorten prematurely. And, since smoking and obesity are associated with heart disease, they wanted to see if there was a connection between heart disease and telomere length. What they found was that if a person’s telomere length is short, then their risk of heart attack and premature death was increased by 50 and 25 percent, respectively. “That smoking and obesity increases the risk of heart disease has been known for a while. We have now shown, as has been speculated, that the increased risk is directly related to the shortening of the protective telomeres—so you can say that smoking and obesity ages the body on a cellular level, just as surely as the passing of time,” says Borge Nordestgaard, co-author of the study and Clinical Professor of Genetic Epidemiology at the University of Copenhagen. A second finding from the study was that one in four Danes possesses telomeres with such short lengths that not only will they statistically die prematurely, but their risk of heart attack is also increased by almost 50 percent. The idea that telomere length may be related to aging is not a new one. Furthermore, at least one study has demonstrated that L-Carnosine may play a protective role in preventing telomere damage and in decreasing the rate of telomere shortening during cell division—which technically is slowing down the aging process. L-Carnosine consists of the two amino acids beta-alanine and histidine, and is found in high concentrations in the muscle and in the brain. L-Carnosine is believed to possess a significant number of powerful antioxidant properties and has been proposed to be a potential anti-aging compound that can reduce wrinkles and fine lines, improve brain functioning and prevent or treat cataracts of the eyes. In fact, in a recent episode of the Dr. Oz Show, Dr. Oz promoted L-Carnosine as a miracle pill for anti-aging that will help a woman feel younger, look younger and see better. He says that as we age, our natural levels of L-Carnosine drop and that by taking a 500 mg supplement twice a day that we can expect to see a marked improvement in our skin within three months. Current medical opinion is that the benefits of supplemental L-Carnosine are questionable and based on scant scientific evidence. One study published in 2004 claims that culturing human lung cells in a tissue culture solution supplemented with L-Carnosine resulted in reducing telomere shortening and extending the lifespan of the cells. Extending tissue culture results with one cell type to that of a human body during a person’s lifetime is a bit of a stretch to say the least, but still—the results are intriguing. In spite of a lack of concrete evidence that L-Carnosine is an anti-aging miracle, it does have health benefits and is used for preventing or treating complications of diabetes such as nerve damage, cataracts and kidney problems. With additional research and a better understanding of how telomere shortening works and its connection to disease, it is possible that scientists may one day discover a way to create an anti-aging miracle pill such as L-Carnosine that will extend our lifespans. |