For anyone who has ever studied the science of how the molecular biology of the growth plate within hormones interact this post will be a complete outline and summary on one of the best well understood hormone/protein pathways. It includes the Parathyroid Hormone-Related Protein (PTHrP), Parathyroid Hormone (PTH), and Indian Hedgehog (Ihh).
Because it is so well understood, it is easy to understand why and how it affects the fate of the growth plates.
Analysis & Interpretation
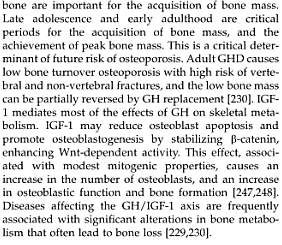
If you treated young mice (not adult mice) with the PTH, growth plate thickness, chondrocyte number, and the overall bone lengthen increases. If you tried to unload the bone, you decrease all three. If you did PTH administration with unloading, the PTH reduces the effect of unloading the bone.
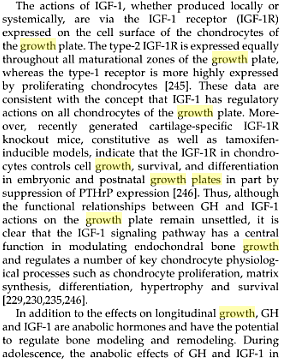
The PTHrP (parathyroid hormone related peptide) and Ihh (indian hedgehog) works in a growth restraining feedback loop by controlling the pace of chondrocyte differentiation in the human growth plate. The PTHrP and Ihh are expressed at the same chondrocytes are the estrogen receptors alpha and beta types. If you remove either the PTH or the PTHrP it results in bone abnormal growth which is usually stunting from the chondrocytes which age faster and multiply slower.
IHH, PTHrP, and PTH/PTHrP receptor mRNA were detected in prehypertrophic and hypertrophic chondrocytes in both sexes during development. The presence of IHH, PTC, PTHrP, and PTH/PTHrP receptor protein in prehypertrophic and hypertrophic chondrocytes. In addition, staining for hedgehog, PTC, and PTHrP also was observed in growth plate stem cells. Furthermore, no mRNA or protein expression of the mentioned factors was detected in the perichondrium. Our data suggest that in contrast to the proposed feedback loop in the early embryonic growth plate, which requires the presence of the perichondrium, a feedback loop in the postnatal growth plate can be confined to the growth plate itself. In fact, two loops might exist: (1) a loop confined to the transition zone and early hypertrophic chondrocytes, which might in part be autocrine and (2) a loop involving the growth plate stem cells.
Indian hedgehog (IHH), produced by prehypertrophic and hypertrophic chondrocytes, stimulates production of parathyroid hormone-related protein (PTHrP) by perichondrial and early chondrocytic cells. PTHrP then maintains chondrocytes in a proliferative, less differentiated state. IHH and PTHrP may participate in a negative feedback loop that synchronizes and determines the pace of differentiation of chondrocytes in the growth plate. IHH is a master regulator of both chondrocyte and osteoblast differentiation.
PTHrP appears to act as a bifunctional modulator of both chondrocyte proliferation and differentiation, through signal transduction linked to the PTH/PTHrP receptor and by its direct action in the nucleolus. While the downstream promoter controls PTH/PTHrP receptor gene expression in bone and cartilage, it is differentially regulated in the two tissues. 1alpha,25-dihydroxyvitamin D3 downregulated the activity of the downstream promoter in osteoblasts, but not in chondrocytes, both in vivo and in vitro
As always the most important parts are highlighted.
From PubMed study HERE
PTHrP and the regulation of longitudinal growth: Exploring novel strategies to increase final height in children with short stature.
Dit is de samenvatting van de aanvraag.
Longitudinal growth is controlled by the activity of the chondrocytes in the epiphyseal plate, which is present at the distal ends of the long bones, and is regulated by a multitude of hormones, growth factors and metabolic conditions. Among these, gonadal steroids and particularly estrogen, are of pivotal importance. In humans, estrogen is responsible for growth plate closure causing a complete arrest in longitudinal growth at the end of puberty. The molecular mechanisms by which estrogen influences longitudinal growth and induces growth plate closure are largely unknown. One such mechanism can be the growth restraining feedback loop consisting of Parathyrooid Hormone related Peptide (PTHrP) and Indian Hedgehog (Ihh). This feedback loop has been shown to regulate longitudinal bone growth by controlling the pace of chondrocyte differentiation in the embryonal chicken, mice and human growth plate. More recently, we have shown that this feedback loop is also operational after birth. In addition, we have found that PTHrP, Ihh and estrogen receptors Era and ß are co-expressed in the same growth plate chondrocytes and that gonadectomy influences the expression of components of the feedback loop. A role for PTHrP in growth plate closure is furthermore supported by observations in humans and transgenic mice, in which the presence of an constitutively activating mutation in the PTH/PTHrP-receptor results in dwarfism and premature growth plate closure. At present, the molecular mechanisms by which the PTHrP/Ihh-feedback loop controls chondrocyte differentiation and is modulated by estrogen are largely unknown. The aims of the present project are: To gain more insight in the molecular mechanism by which ethe PTHrP/Ihh feedback loop controls the pace of chondrocyte differntiation with empasis on the identification of PTHrP target genes and interactions with members of the TGFß/BMP-superfamily. To characterize the interactions between the PTHrP/Ihh-feedback loop and estrogen and to determine its role in growth plate closure. To examine the relevance of the findings in experimental models for longitudinal growth and in archival material of human growth plates. In vitro and ex vivo experiments are performed using the chondrogenic cell line ATDC-5 and cultured metatarsals of wild type, PTHrP -/- and PTH/PTHrP-receptor -/- mice. PTHrP target genes will be identified using DNA microarray technology using both commercially available and custom made cDNA chips. Adenoviral mediated gene transfer will be used to manipulate the expression of receptor for members of the TGFß/BMP-superfamily and estrogen (e.g. mock, wild type, constituteively active, dominant negative). The effects of tese manipulations on the PHTrP/Ihh-feedback loop will be examined at the mRNA level using custom made cDNA chips, at the level of signal transduction by studying the activation state of cytoplasmic signal transduction intermediates, and at the level of transcriptional activation using artificial promotor reporter constructs. The relevance of the findings will be tested in previously collected epiphyseal plates of gonadectomized growing rats or of immature rats supplemented with various sex-steroieds. This material is well characterized with respect to various parameters regarding growth allowing direct correlations between the expression of the candidate tgenes and longitudinal growth. Furthermore, findings will be extended to the human situation by analysing archival growth plates of lethal skeletal dysplasias and of children who have undergone orthopaedic surgery. The results obtained in this study will extent our knownledge on the molecular mechanisms by which longitudinal growth is regulated at the level of the epiphyseal plate and growth plate closure is induced. As such the results will help in the rational design of novel diagnostic tests and growth promoting strategies for growth retarded patients who do not benefit from currently available therapies.
From PubMed study HERE
Journal of Bone and Mineral Metabolism
March 2002, Volume 20, Issue 2, pp 83-90
Human PTH (1–34) induces longitudinal bone growth in rats
Taishi Ogawa, Hiroshi Yamagiwa, Tadashi Hayami, Zhang Liu, Kuan-Yu Huang, Kunihiko Tokunaga,Takehiro Murai, Naoto Endo
Abstract.
The growth plate is a specialized structure that is responsible for longitudinal bone growth (LGR). Growth plate organization is altered with loading in rats. Parathyroid hormone (PTH) is known to induce mitogenic effect on chondrocytes in vitro. Type I PTH/PTH related peptide (rP) receptor is expressed in growth plate cartilage in rats. We therefore investigated the effect of PTH administration on the organization and longitudinal growth rate of the growth plate in rats. We also investigated the effect of PTH on the changes induced by unloading in the organization and growth of the growth plate. Thirty 6-week-old and 30 15-week-old male Sprague-Dawley rats were randomly assigned to five groups (n = 6 per group), i.e., basal controls, control (i.e., normally loaded), PTH-treated control (i.e., PTH-treated under normal loading), unloaded, and PTH-treated under unloading. PTH-treated animals received human PTH (1–34) at a dose of 80 μg/kg per day five times per week for 3 weeks, for the duration of unloading. In young loaded rats treated with the systemic administration of PTH, growth plate thickness, chondrocyte number, and LGR were increased in the proximal tibiae compared with findings in young loaded rats without PTH administration. Hindlimb unloading induced a reduction in growth plate thickness, chondrocyte number, and LGR. In young rats, systemic administration of PTH partly prevented these changes induced by unloading. These preventive effects of PTH were observed only in young rats; not in adult rats. These results show that the systemic administration of PTH stimulates longitudinal bone growth, and diminishes the reduction in growth plate growth induced by unloading in young rats.
From PubMed study HERE
Histol Histopathol. 2000 Jul;15(3):957-70.
Recent studies on the biological action of parathyroid hormone (PTH)-related peptide (PTHrP) and PTH/PTHrP receptor in cartilage and bone.
Amizuka N, Henderson JE, White JH, Karaplis AC, Goltzman D, Sasaki T, Ozawa H.
Source
Department of Oral Anatomy, Niigata University Faculty of Dentistry, Japan. amizuka@dent.niigata-u.ac.jp
Abstract
Mice with a targeted deletion of parathyroid hormone (PTH)-related peptide (PTHrP) develop a form of dyschondroplasia resulting from diminished proliferation and premature maturation of chondrocytes. Abnormal, heterogeneous populations of chondrocytes at different stages of differentiation were seen in the hypertrophic zone of the mutant growth plate. Although the homozygous null animals die within several hours of birth, mice heterozygous for PTHrP gene deletion reach adulthood, at which time they show evidence of osteopenia. Therefore, PTHrP appears to modulate cell proliferation and differentiation in both the pre and post natal period. PTH/PTHrP receptor expression in the mouse is controlled by two promoters. We recently found that, while the downstream promoter controls PTH/PTHrP receptor gene expression in bone and cartilage, it is differentially regulated in the two tissues. 1alpha,25-dihydroxyvitamin D3 downregulated the activity of the downstream promoter in osteoblasts, but not in chondrocytes, both in vivo and in vitro. Most of the biological activity of PTHrP is thought to be mediated by binding of its amino terminus to the PTH/PTHrP receptor. However, recent evidence suggests that amino acids 87-107, outside of the amino terminal binding domain, act as a nucleolar targeting signal. Chondrocytic cell line, CFK2, transfected with wild-type PTHrP cDNA showed PTHrP in the nucleoli as well as in the secretory pathway. Therefore, PTHrP appears to act as a bifunctional modulator of both chondrocyte proliferation and differentiation, through signal transduction linked to the PTH/PTHrP receptor and by its direct action in the nucleolus.
PMID: 10963138 [PubMed – indexed for MEDLINE]
From PubMed study HERE
The parathyroid hormone-related protein and Indian hedgehog feedback loop in the growth plate.
Kronenberg HM, Chung U.
Source
Endocrine Unit, Massachusetts General Hospital and Harvard Medical School, Boston, MA 02114, USA.
Abstract
Normal development of the growth plate requires coordinated proliferation and differentiation of chondrocytes and osteoblasts. In previous work, we have shown that Indian hedgehog (IHH), produced by prehypertrophic and hypertrophic chondrocytes, stimulates production of parathyroid hormone-related protein (PTHrP) by perichondrial and early chondrocytic cells. PTHrP then maintains chondrocytes in a proliferative, less differentiated state. Because this less differentiated state delays the production of IHH, IHH and PTHrP may participate in a negative feedback loop that synchronizes and determines the pace of differentiation of chondrocytes in the growth plate. To establish the roles of physiological levels of PTHrP and IHH, we have now injected PTH/PTHrP receptor (-/-) embryonic stem (ES) cells into normal blastocysts to generate mice with chimeric growth plates. The PTH/PTHrP receptor cells leave the proliferative cycle and differentiate prematurely in the middle of the normal proliferative columns. The columns of wild-type cells are longer than normal and the adjacent bone collar is also longer than normal. Patterns of gene expression and the use of chimeras using PTH/PTHrP receptor (-/-); IHH (-/-) ES cells suggest that modified patterns of IHH and PTHrP synthesis explain these abnormalities. Thus, IHH is a master regulator of both chondrocyte and osteoblast differentiation.
PMID: 11277077 [PubMed – indexed for MEDLINE]
From PubMed study HERE
J Bone Miner Res. 2000 Jun;15(6):1045-55.
Expression of Indian hedgehog, parathyroid hormone-related protein, and their receptors in the postnatal growth plate of the rat: evidence for a locally acting growth restraining feedback loop after birth.
van der Eerden BC, Karperien M, Gevers EF, Löwik CW, Wit JM.
Source
Department of Pediatrics, Leiden University Medical Center, The Netherlands.
Abstract
A locally acting growth restraining feedback loop has been identified in the murine embryonic growth plate in which the level of parathyroid hormone-related peptide (PTHrP) expression regulates the pace of chondrocyte differentiation. To date, it is largely unknown whether this feedback loop also regulates the pace of chondrocyte differentiation in the growth plate after birth. We therefore characterized the spatio-temporal expression of Indian hedgehog (IHH), PTHrP, and their receptors in the postnatal growth plate from female and male rats of 1, 4, 7, and 12 weeks of age. These stages are representative for early life and puberty in rats. Using semiquantitative reverse-transcription polymerase chain reaction (RT-PCR) on growth plate tissue, IHH and components of its receptor complex, patched (PTC) and smoothened (SMO), PTHrP and the type I PTH/PTHrP receptor messenger RNA (mRNA) were shown at all ages studied irrespective of gender. Using in situ hybridization, IHH, PTHrP, and PTH/PTHrP receptor mRNA were detected in prehypertrophic and hypertrophic chondrocytes in both sexes during development. In addition, especially in the younger age groups, faint expression of PTH/PTHrP receptor mRNA also was shown in stem cells and proliferative chondrocytes. Immunohistochemistry confirmed the observations made with in situ hybridization, by showing the presence of IHH, PTC, PTHrP, and PTH/PTHrP receptor protein in prehypertrophic and hypertrophic chondrocytes. In addition, staining for hedgehog, PTC, and PTHrP also was observed in growth plate stem cells. No differences in staining patterns were observed between the sexes. Furthermore, no mRNA or protein expression of the mentioned factors was detected in the perichondrium. Our data suggest that in contrast to the proposed feedback loop in the early embryonic growth plate, which requires the presence of the perichondrium, a feedback loop in the postnatal growth plate can be confined to the growth plate itself. In fact, two loops might exist: (1) a loop confined to the transition zone and early hypertrophic chondrocytes, which might in part be autocrine and (2) a loop involving the growth plate stem cells.
PMID: 10841173 [PubMed – indexed for MEDLINE]