Me: After going back and reading on my old posts on the power of possibly using BMPs to induce chondrocytes and also heal and regenerate articular cartilage, I have created a new idea on how it might be possible to induce longitudinal growth using a combination of at least 3 main ideas I have been exploring for the last 3 months. This a culmination of all of my learning and research so far. You can see from another post way back entitled” Increase Height Through Surgical Method By Cartilage Harvesting And Chondrocyte Implantation With Growth Factor Injections” that I tried also at that time to think up a possible height increase less invasive surgical path as well, but this new method is more likely to work out and the procedure is more detailed, due to new understanding and research.
Note: One of the biggest issues we have to understand is what exactly are the differences between the articular cartilage found at the end of the long bones and the original growth plates we had. Remember that both of these cartilage ar hyaline to begin with. The major differences are…
1. The epiphyseal has a perichondrium, the articular does not.
2. The epiphyseal cartilage is created from two centers of ossification, a primary and secondary. The articular cartilage does not. This means that the epiphyseal is directly connected to layers of trabecular bone which house progenitor cells on both sides. The articular does not.
3. The epiphyseal has a resting zone area, the articular does not.
One idea I originally thought about is try to get the articular cartilage to behave as much like the growth plates as possible.
Note: The other big thing we would have to get right is the right growth factor combination. At this point I am guessing to use BMP-7 (obviously), TGF-Beta2 & 3 (since there have been articles that showed that BMPs need it to work out), BMP-2 since it is the only other BMP that leads to chondorgenesis. We want to avoid BMPs which give better results for osteogenesis but have shown to result in chondrogenesis. The other big one I wanted to use was GDFs which are growth differentiation factors.
So Step 1…
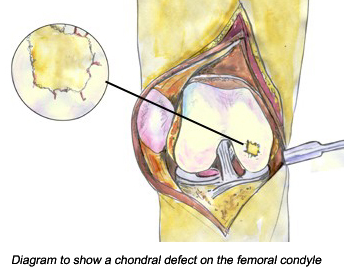
You create two chondral defects through the knee which connects the inner epiphysis trabecular bone with the articular cartilage. This can be done through two holes punctures through the cartilage of a bend knee (from diagram above). The puncture can be just 2-3 mm in diameter (from study 1). I would suggest using a diameter of 4-5 mm if that is possible. The exact location the punctures will go through will be the trochlea and the femoral condyle (from study 2). The penetration will be through the hard bone, into the epiphysis until it reaches the axis distance where the original epiphyseal plates will be (from X-ray).
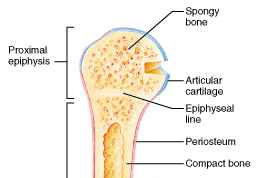 Note: From this diagram on the right it suggest that even after the epiphyseal line is gone, there seems to be still a band of trabecular bone which might be slightly thicker and denser in the area where the epiphysis and metaphysis are connect than in other areas in the inner bone. That is the line you want to reach, but right before you hit that thick band of dense bone.
Note: From this diagram on the right it suggest that even after the epiphyseal line is gone, there seems to be still a band of trabecular bone which might be slightly thicker and denser in the area where the epiphysis and metaphysis are connect than in other areas in the inner bone. That is the line you want to reach, but right before you hit that thick band of dense bone.
Step 2….
You implant a type of capsule which has a specific mixture of growth factors that focuses on chondrogenesis, not osteogenesis. The growth factors can be encapsulated in a collagen and proteoglycan mixture. It is essentially cartilage matrix with growth factors inside but no chondrocytes. The mixture will have BMP-7 and BMP-2 which has shown to help differentiate the progenitor cells in the right way to give you the type of cell desired . You want to avoid types of growth factors which are mainly osteogenic.
We know that adult marrow is mostly yellow marrow of adiposytes and fatty acids. Study 5 suggest using instead BMP-6 and TGF-Beta 3 to convert them into chondrocytes. Remember that the bones are one of the tissues that regenerate and heal the fastest. That means that one must focus less on bone healing and more on how to get the cartilage to expand.
Step 3…
After the implant capsule of growth factors, you implant another capsule that has chondrocytes encased surrounded by a perichondrium layer. This is actual growth plate cartilage grown in vitro. I have researched one study that has shown that in vitro growth plates have been developed and can be reapplied back to function properly in vivo. Study 3 showed that chondorcytes can be differentiated from using perichondrium with BMP-7 so this step should be easy.
Step 4…
You make a cut around the outer edge of the long bone right at the distance where the initial penetration was done. You remove 3-4 mm in thickness axially of outer bone which will be the periosteum but also 4-5 cm radially inward. This is to remove the outer hard cortical bone. The other reason you want it to be at least this thick, is to prevent bone repair. If the created fracture was any thinner the bones will automatically heal over it. You implant resting zone progenitor cells in these locations which are surrounded in cartilage which is themselves surrounded by perichondrium.
Step 5…
A type of gause wrapping with BMP-7 and other growth factors in it which promote chondrogenesis is wrapped around the incision location and the cartilage implant. This has two functions. One is to prevent the cartilage inside the epiphysis to push out laterally. The other is to be a source of more growth factor to get the outer cartilage implant to push inward.
Step 6…
You fill up the original knee chondral defect first using a type of ostegenic BMP like 6 or 9 with TGF-Beta 3 (study 5), and later use a chondrogenic growth factor like BMP-7 which has shown to regrow the articular cartilage (from study 2).
Step 7…
What this method have effectly done is removed every single contraint the long bone has which would restrict longitudinal growth. We learned that the compressive strength of long bone is from the calcium hydroxylapatite in the inorganic matric which leads to the hard dense cortical bone to have a youngs modulus yield of upwards of 150 MPa. The tensile strength is from collagen fibrils arranged in lamellae (source is from previous post HERE). This is around 100 MPa. Since step 4 means the hardest material is fractured at a level which the body’s natural osteogenetic, most of the high value of young’s modulus tensile strength from the intact long bone will be gone. It will be just the trabecular bone which is holding the bone attached in that location and that can be stretched relatively easily by the chondrocytes if they go through hypertrophy.
If we remember, the way that the original ilizarov method works, and how distraction osteogenesis works in general is from callus distraction, which means you pull the trabecular bones apart over time. What is great about this method is that Since step 4 is done in the thicker area, you should not need the fixator to hold things stable unlike the ilizarove method, where the fracture created makes the long bone very weak and can break in half since the fracture was made around the middle (or diaphysis) of the long bone. This is one of the reasons you needed an external fixator to keep things stable and fixed so the inner bone which you had not separated wouldn’t move, causing deformities.
Step 8…
What you have done is basically created an entirely new growth plate similar to what the body’s original prenatal ossification development was like. Remember that the growth plate was formed from two primary ossification centers, My idea is to create two places which sort of goes through chondrocyte proliferation embedding into the bone matrix in the ossification process which will eventually come together fusing into another growth plate in the inside of the lone bone which is around the same location as the natural one.
If you have studied physics you might remember the calculations you have had to do for electrical fields or magnetic fields of a type of 3 dimensional body form called an annulus. I am treating the long bone as a concentric annulus. where the outer most circular region has cartilage implantation and the inner circle has cartilage implantation. The growth factors from both the inside implant and the outside implant will diffuse into the surrounding bones turning any adult fatty acid marrow MCSs into chondrocytes. If that is sustained, the osteoclasts can clean up most of the dead bone lucanae. If the calcium crystals are hard to remove, we can swing the PTH/TH control so that calcium can be removed from the bones and goes back into the blood stream.
Any expansion the implanted cartilage will do have to move to the state or postion of lowest resistance. Since the lowest resistance is the area where you have made a fracture and implanted cartilage, the inner material will get pushed out. Since you are holding the outer edges intact, the force of pressure will push in the longitudinal direction. since the edges of the outer fracture will be moving away from each other.
Sorry if I have no pictures to explain what I mean.
From PubMed study 1 HERE…
Br J Oral Maxillofac Surg. 2002 Jun;40(3):201-6.
Regeneration of defects in the articular cartilage in rabbit temporomandibular joints by bone morphogenetic protein-2.
Source
Department of Oral and Maxillofacial Surgery, Kanazawa Medical University, Ishikawa, Japan.
Abstract
The purpose of this study was to investigate the therapeutic use of recombinant human bone morphogenetic protein-2 (rhBMP-2) in internally deranged temporomandibular joints (TMJ). Defects (2 mm in diameter) were created in the surface of the condylar head. Lyophilized rhBMP-2 with collagen as the carrier was implanted in the defects in different doses: rhBMP-2 15 microg (n = 5); rhBMP-2 3 microg (n = 5); rhBMP-2 0.6 microg (n = 5). In the two control groups, the defects were either filled with collagen alone (n = 5) or left untreated (n = 5). Three weeks postoperatively the sites of defects were examined under light microscopy. In the 15 micromg and the 3 microg groups, new cartilage had filled the defects; endochondral ossification was also found deep within the defect. In the 0.6 microg group, fibrous tissue was proliferating in most areas of the defect, although cartilage was also found in some parts. In the two control groups, there was either soft tissue repair only or no evidence of tissue repair. These findings suggest that BMP-2 could stimulate the repair of defects in the articular cartilage of the mandibular condyle head during the 3 weeks postoperatively. To observe the progress of endochondral ossification in more detail, it may be necessary to extend the experiment for a longer period of time. However, this study supports the contention that BMP-2 may be useful in the regeneration of cartilage in TMJ disease.
Copyright 2002 The British Association of Oral and Maxillofacial Surgeons.
- PMID: 12054709 [PubMed – indexed for MEDLINE]
- From PubMed study HERE…
- Growth Factors. 2001;19(2):101-13.
Regeneration of articular cartilage chondral defects by osteogenic protein-1 (bone morphogenetic protein-7) in sheep.
Source
Department of Anatomy, School of Medicine, University of Zagreb, Croatia.
Abstract
The efficacy of osteogenic protein-1 (OP-1; BMP-7) in regeneration of articular cartilage was examined by creating knee chondral defects in sheep. With a specially designed instrument in both knees, two 10 mm (diameter) chondral defects were created: one in the trochlea and the other on the femoral condyle. The recombinant BMP was delivered via an extra-articulary positioned mini-osmotic pump, which was fixed to the femoral diaphysis above the knee joint, and connected by a polyethylene tubing to the articular space. Prior to use, the compatibility of OP-1 with mini-osmotic pumps was tested in vitro by measuring aggregation/precipitation and modification of the released protein by size exclusion and reversed phase HPLC. The average amount of aggregation was 15% and about 5% of OP-1 was modified. However, the biological activity of OP-1 released from pumps over a period of 2 weeks at 37 degrees C was equal to ROS cell assay OP-1 standard. Following surgery, a total of 55 microg (low dose) or 170 microg (high dose) OP-1 in acetate buffer (pH 4.5) was slowly released from the pump over a period of 2 weeks. The pumps connected to control knees were filled with acetate buffer as a vehicle. Twelve animals were operated, six of which were treated with the low OP-1 dose, and six with the high OP-1 dose. Three sheep of each group were killed either at 3 or 6 months following surgery, based on arthroscopical evaluation. The chondral defects in the control knees remained empty during the observation period. At 3 months following surgery, defects treated with both OP-1 doses were filled with connective tissue and cartilage. At 6 months following surgery, both doses of OP-1 stimulated regeneration in treated knees. The boundaries between new and old cartilage were well fused and mechanically resisted animals’ weight bearing. The regenerated cartilage was rich in proteoglycans and type II collagen, as demonstrated by toluidine blue staining and immunohistochemistry. No signs of endochondral bone formation above the bony tidemark were observed. We suggest that a recombinant bone morphogenctic protein stimulates ingrowth of mesenchymal cells into the chondral defects which then transform into newly formed articular cartilage-like tissue.
- PMID: 11769970 [PubMed – indexed for MEDLINE]
- From PubMed study 3 HERE…
- Tissue Eng. 1998 Fall;4(3):305-13.
Stimulation of cartilage differentiation by osteogenic protein-1 in cultures of human perichondrium.
Source
Department of Oral Cell Biology, ACTA-Vrije Universiteit, 1081 BT Amsterdam, The Netherlands.
Abstract
Exposure of progenitor cells with chondrogenic potential to recombinant human osteogenic protein-1 [rhOP-1, or bone morphogenetic protein-7 (BMP-7] may be of therapeutic interest in the regeneration of articular cartilage. Therefore, in this study, we examined the influence of rhOP-1 on cartilage formation by human perichondrium tissue containing progenitor cells with chondrogenic potential in vitro. Fragments of outer ear perichondrium tissue were embedded in clotting autologous blood to which rhOP-1 had been added or not (controls), and the resulting explant was cultured for 3 weeks without further addition of rhOP-1. Cartilage formation was monitored biochemically by measuring [³5;S]sulfate incorporation into proteoglycans and histologically by monitoring the presence of metachromatic matrix with cells in nests. The presence of rhOP-1 in the explant at the beginning of culture stimulated [³5;S]sulfate incorporation into proteoglycans in a dose-dependent manner after 3 weeks of culture. Maximal stimulation was reached at 40 microgram/ml. Histology revealed that explants treated with 20-200 microgram/ml rhOP-1, but not untreated control explants, contained areas of metachromatic-staining matrix with chondrocytes in cell nests. These results suggest that rhOP-1 stimulates differentiation of cartilage from perichondrium tissue. The direct actions of rhOP-1 on perichondrium cells to stimulate chondrocytic differentiation and production of cartilage matrix in vitro provide a cellular mechanism for the induction of cartilage formation by rhOP-1 in vivo. Thus, rhOP-1 may promote early steps in the cascade of events leading to cartilage formation. Therefore, rhOP-1 could be an interesting factor for regeneration of cartilage in articular cartilage defects.
- PMID: 9836793 [PubMed – indexed for MEDLINE]
- From PubMed study 4 HERE
- Osteoarthritis Cartilage. 2002 May;10(5):394-401.
Cartilage-derived morphogenetic protein-1 and -2 are endogenously expressed in healthy and osteoarthritic human articular chondrocytes and stimulate matrix synthesis.
Source
Department of Rheumatology, Internal Medicine III, University of Vienna, Austria.
Abstract
OBJECTIVE:
We investigated whether chondrocytes derived from osteoarthritic cartilage may lose their responsiveness to cartilage-derived morphogenetic protein-1, -2 (CDMP-1, -2) and osteogenic protein-1 (OP-1) compared with healthy cells, thus leading to an impaired maintenance of matrix integrity.
DESIGN:
Chondrocytes were isolated from articular cartilage from patients with and without osteoarthritic lesions. Cells were grown as monolayer cultures for 7 days in a chemically defined serum-free basal medium (BM) in the presence of recombinant CDMP-1, -2, and OP-1. Glycosaminoglycan synthesis was measured by [35S]Sulfate incorporation into newly synthesized macromolecules. Cell proliferation was investigated by [3H]Thymidine incorporation. The endogenous gene expression of CDMPs/OP-1 and their respective type I and type II receptors was examined using RT-PCR. The presence of CDMP proteins in tissue and cultured cells was detected by Western immunoblots.
RESULTS:
mRNAs coding for CDMPs and their respective receptors are endogenously expressed not only in healthy, but also in osteoarthritic cartilage. CDMP proteins are present in both normal and osteoarthritic articular cartilage and cultured chondrocytes. CDMP-1, CDMP-2 and OP-1 markedly increased glycosaminoglycan synthesis in both healthy (P< 0.01) and osteoarthritic (P< 0.05) human articular chondrocytes. A comparison of the glycosaminoglycan biosynthetic activity between healthy and osteoarthritic samples revealed no detectable difference, neither in stimulated nor in unstimulated cultures. [(3)H]Thymidine incorporation showed that CDMPs/OP-1 did not affect cell proliferation in vitro.
CONCLUSION:
CDMPs and OP-1 exert their anabolic effects on both healthy and osteoarthritic chondrocytes indicating no loss in responsiveness to these growth factors in OA. The endogenous expression of CDMPs/OP-1 and their receptors suggest an important role in cartilage homeostasis.
Copyright 2002 OsteoArthritis Research Society International. Published by Elsevier Science Ltd. All rights reserved.
- PMID: 12027540 [PubMed – indexed for MEDLINE]
- From PubMed study 5 HERE
- Tissue Eng Part B Rev. 2010 Aug;16(4):435-44.
Comparative review of growth factors for induction of three-dimensional in vitro chondrogenesis in human mesenchymal stem cells isolated from bone marrow and adipose tissue.
Source
Joint Department of Biomedical Engineering at UNC-Chapel Hill and North Carolina State University, Raleigh, North Carolina 27695, USA.
Abstract
The ability of bone-marrow-derived mesenchymal stem cells (MSCs) and adipose-derived stem cells (ASCs) to undergo chondrogenic differentiation has been studied extensively, and it has been suggested that the chondrogenic potential of these stem cells differ from each other. Here, we provide a comprehensive review and analysis of the various growth factor induction agents for MSC and ASC three-dimensional in vitro chondrogenic differentiation. In general, the most common growth factors for chondrogenic induction come from the transforming growth factor beta (TGFbeta) superfamily. To date, the most promising growth factors for chondrogenesis appear to be TGFbeta-3 and bone morphogenetic protein (BMP)-6. A thorough review of the literature indicates that human MSCs (hMSCs) appear to exhibit the highest chondrogenic potential in three-dimensional culture in the medium containing both dexamethasone and TGFbeta-3. Some reports indicate that the addition of BMP-6 to TFGbeta-3 and dexamethasone further increases hMSC chondrogenesis, but these results are still not consistently supported. Induction of human ASC (hASC) chondrogenesis appears most successful when dexamethasone, TGFbeta-3, and BMP-6 are used in combination. However, to date, current formulations do not always result in stable differentiation to the chondrocytic lineage by hMSCs and hASCs. Continued research must be performed to examine the expression cascades of the TFGbeta superfamily to further determine the effects of each growth factor alone and in combination on these stem cell lines.
- PMID: 20196646 [PubMed – indexed for MEDLINE]

interesting… I always found that the BMPS were very important.
They are. It seems the key is figuring out which BMPs are better for chondrogenesis then osteogenesis. Osteogenesis is easy since the bones are the quickest to repair in terms of types of tissue. Your study on Statin showed that. It is the creation of chondrocytes and cartilage that is hard.
dear sir how to increase height in which nutrition we are eating there for we grow talller do you have any idea