Me: I think this study that I somehow found seems to bring up evidence that the LSJL method that some height increase seekers have been using might not be effective in generating the gains they have been hoping for. While the study never states at face value that there was at metaphysis lengthening form loading in prepubertal girls as compared to post pubertal girls, it seems to suggestively imply that.
The issue is that with the study, I couldn’t find where they said what actual type of loading was done. Did they use the same type of device as the stuff used on mice with Yokota and Zhang?
Analysis: The thing about this study was that length was never measured but only cortical diameter. As stated from the results section “Growth itself, as reflected by structural changes in the nonloaded arm, resulted in a 14% increase in cortical area of the mid- and distal humerus from the pre- to peripubertal years because of greater periosteal expansion than medullary expansion“. That could correlate to length increase assuming proportional size increase. For my final interpretation, I am of course assuming that the periosteal appositional growth we see in prepubertal females don’t just affect in the radial direction leading to bone thickening but also the limb ends of the epiphysis which leads to long bone lengthening too. For the post pubertal females the endocortical surface is what is begin affected, which means nothing to the outer surface of the bone which means neither lengthening to shortening.
Main Points: “Cortical areas of the mid- and distal humerus were ∼14% greater in the peripubertal players than in the prepubertal players because of greater periosteal expansion than medullary expansion. Cortical areas of the mid- and distal humerus were ∼20% greater in the postpubertal players than in the peripubertal players. At the midhumerus, this was the result of periosteal expansion alone, whereas at the distal humerus, medullary contraction contributed to the larger cortical area”
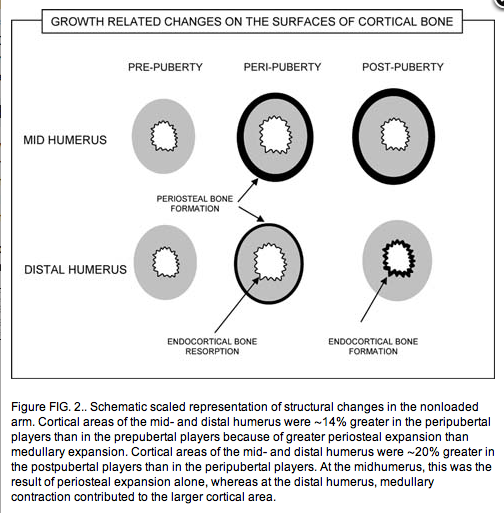
Before puberty, periosteal apposition accounts for most of the increase in cortical area. Endocortical resorption creates an enlarging marrow cavity and partly offsets the increase in cortical area produced by periosteal apposition. The net result is an enlarged cortical area located further from the neutral axis, which has the effect of increasing its resistance to bending.(3) Late in puberty, periosteal apposition continues with a contribution from endocortical apposition(7); particularly at the distal humerus, where, in this study, the increase in cortical area was the result of equal contributions from periosteal and endocortical apposition.
Before puberty, loading magnified periosteal apposition. During the postpubertal period, loading magnified the effect of endocortical apposition, which makes an important contribution to cortical thickness in females. Indeed, endocortical apposition accounted for most of the greater side-to-side difference attained in the postpubertal years.
Thus, loading affects both the periosteal and the endocortical surfaces but the magnitude of the effects vary according to whether the surface is anterior, posterior, medial, or lateral and according to whether the region is proximal, central, or distal along the bone’s length.
In conclusion, loading before puberty increases bone size and its resistance to bending. After puberty, loading increases the acquisition of bone on the endocortical surface with little benefit in the bone’s resistance to bending. Growth and the effects of loading were surface specific and varied along the length of the bone depending on the maturation of the region as well as the intensity and direction of loading. Increasing the bone’s resistance to bending and torsion is achieved by modifying the shape and mass of bone but not necessarily its density.
Conclusion: What might be the smartest thing to do is actually try to contact the people who originally did this experiment and try to ask them whether they ever tried to collect length data for the unloaded/loaded differences.
From PubMed Source link HERE…
The effect of mechanical loading on the size and shape of bone in pre-, peri-, and postpubertal girls: a study in tennis players.
Source
School of Health Sciences, Deakin University, Melbourne, Australia.
Abstract
Exercise during growth results in biologically important increases in bone mineral content (BMC). The aim of this study was to determine whether the effects of loading were site specific and depended on the maturational stage of the region. BMC and humeral dimensions were determined using DXA and magnetic resonance imaging (MRI) of the loaded and nonloaded arms in 47 competitive female tennis players aged 8-17 years. Periosteal (external) cross-sectional area (CSA), cortical area, medullary area, and the polar second moments of area (I(P), mm4) were calculated at the mid and distal sites in the loaded and nonloaded arms. BMC and I(P) of the humerus were 11-14% greater in the loaded arm than in the nonloaded arm in prepubertal players and did not increase further in peri- or postpubertal players despite longer duration of loading (both, p < 0.01). The higher BMC was the result of a 7-11% greater cortical area in the prepubertal players due to greater periosteal than medullary expansion at the midhumerus and a greater periosteal expansion alone at the distal humerus. Loading late in puberty resulted in medullary contraction. Growth and the effects of loading are region and surface specific, with periosteal apposition before puberty accounting for the increase in the bone’s resistance to torsion and endocortical contraction contributing late in puberty conferring little increase in resistance to torsion. Increasing the bone’s resistance to torsion is achieved by modifying bone shape and mass, not necessarily bone density.
- PMID: 12469922 [PubMed – indexed for MEDLINE]
This source link HERE is the full article.
Keywords:
- exercise; growth and development; bone strength; rigidity
Abstract
Exercise during growth results in biologically important increases in bone mineral content (BMC). The aim of this study was to determine whether the effects of loading were site specific and depended on the maturational stage of the region. BMC and humeral dimensions were determined using DXA and magnetic resonance imaging (MRI) of the loaded and nonloaded arms in 47 competitive female tennis players aged 8–17 years. Periosteal (external) cross-sectional area (CSA), cortical area, medullary area, and the polar second moments of area (IP, mm4) were calculated at the mid and distal sites in the loaded and nonloaded arms. BMC and IP of the humerus were 11–14% greater in the loaded arm than in the nonloaded arm in prepubertal players and did not increase further in peri- or postpubertal players despite longer duration of loading (both, p < 0.01). The higher BMC was the result of a 7–11% greater cortical area in the prepubertal players due to greater periosteal than medullary expansion at the midhumerus and a greater periosteal expansion alone at the distal humerus. Loading late in puberty resulted in medullary contraction. Growth and the effects of loading are region and surface specific, with periosteal apposition before puberty accounting for the increase in the bone’s resistance to torsion and endocortical contraction contributing late in puberty conferring little increase in resistance to torsion. Increasing the bone’s resistance to torsion is achieved by modifying bone shape and mass, not necessarily bone density.
INTRODUCTION
Exercise during growth results in biologically important increases in bone mass. Growth in bone width and cortical thickness before puberty occurs by greater periosteal (outer surface) apposition than by endocortical (inner surface) resorption. During puberty, estrogen production inhibits periosteal apposition but stimulates the acquisition of bone on the endocortical surface.(1)
It has been proposed that exercise will enhance formation at the surfaces of bone undergoing bone apposition.(2) Because apposition of bone on the periosteal surface is a more effective means of increasing the bending and torsional strength of bone than acquisition of bone on the inner surface,(3) exercise regimens may be more effective when undertaken at a time when the growth of bone is dominated by periosteal rather than endocortical growth.
Exercise has been reported to enhance periosteal expansion in young animals and endocortical contraction in mature animals. Thus, to determine whether the effects of exercise depend on the maturational stage of the region exposed to loading as well as the intensity and duration of the loading, we tested the following hypotheses: (i) loading of bone during tennis playing will result in increased cortical area of the playing humerus because of periosteal expansion with no endocortical apposition in the pre- and peripubertal years; (ii) during the postpubertal years, loading will increase cortical area by endocortical apposition with less contribution from periosteal apposition; and (iii) the exercise-induced increase in cortical area and bending strength of the humerus will be caused by greater periosteal apposition with little or no contribution from endocortical bone acquisition.
MATERIALS AND METHODS
Subjects
Forty-seven pre-, peri-, and postpubertal competitive female tennis players aged 8–17 years were recruited from tennis clubs in Melbourne, Australia. Players were included if they had been playing competitive tennis for a minimum of 2 years and were currently playing at least 3 h/week (Table 1). Forty girls were right-handed, and 41 girls used a double-handed backhand. Longitudinal data were collected in 37 subjects after 1.1 ± 0.01 years (range, 0.8–1.5 years); 6 subjects remained prepubertal, 6 subjects became peripubertal, 9 subjects remained peripubertal, and 16 subjects remained postpubertal during the observation period. Ten subjects were not included because they were either no longer playing (n = 2), not willing to participate (n = 4), or relocated (n = 4).
All girls were healthy and received no medication known to affect the skeleton. The Deakin University and Alfred Hospital ethics committees approved the study, and written consent was obtained from all participants and their parents. Sexual maturation was self-assessed with parental guidance using the standard five-scale Tanner stages for breast development. Subjects were classified as prepubertal (Tanner stage 1), peripubertal (Tanner stage 2–4), or postpubertal (postmenarche).
Bone geometry, mass, and strength
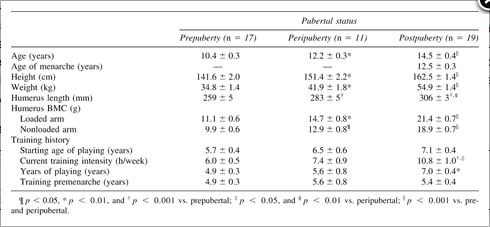
Magnetic resonance imaging (MRI) was used to determine bone dimensions (1.5 T whole-body unit; with a commercial transit-receive torso coil; Signa Advantage GE Medical Systems, Milwaukee, WI, USA). T1-weighted spin-echo images at a repetition time (TR) of 600 ms and an echo time (TE) of 14 ms were acquired in the axial plane. Field of view was 200 mm2 and the matrix size was 512 × 192. The region of interest (ROI) was 30–60% from the distal end of the humerus and was divided into thirds. Areas of the proximal third of the ROI representing the midportion of the humerus were compared with the distal third. Five-millimeter slices (with 5-mm gaps between slices) were scanned along the ROI. Each axial image was analyzed using the OSIRIS imaging software program (Digital Imaging Unit, Center of Medical Informatics, University Hospital of Geneva, Geneva, Switzerland). Periosteal area was the external size of the bone (i.e., periosteal border) and cortical area was periosteal minus the medullary area (Fig. 1).
Figure FIG. 1.. Typical MRI transverse slices of cortical bone (black) and medullary area (white) of the playing and nonplaying humerus of a postpubertal female tennis player. ROIs were analyzed at the mid- and distal humerus, each representing 10% of the total arm length (30–40% and 50–60%, measured from the distal condyles).
Summing the cross-sectional areas of each slice in the ROI divided by the total number of slices in the ROI determined average periosteal, cortical, and medullary areas. The short-term precision (CV) was 1.02% and 0.21% for periosteal and cortical bone areas, respectively. In vivo studies using bovine bones have shown that MRI provides accurate estimates of bone cross-sectional areas, and these data correlate well with quantitative computed tomography (QCT) measurements of the same bone (r2 = 0.98).(4) Bone mineral content (BMC) of the playing and nonplaying arm was measured using DXA (CV for BMC was 3.6%; Lunar DPX-L, version 1.3b; Lunar Corp., Madison, WI, USA).
To assess the bones resistance to bending (rigidity), each image was imported into Scion Image 4.0.2 (Scion Corp., Frederick, MD, USA). The maximum (IMAX, mm4) minimum (IMIN, mm4), and polar (IP, mm4) second moments of area were calculated using a custom macro. The second moment of area (I) reflects a structure’s resistance to bending and is calculated by dividing the section into small areas (pixels), and multiplying each (dA) by its squared distance from the neutral plane. This procedure is integrated over the entire cross-section. The macro calculates I about all possible neutral planes and reports the largest value as IMAX and the smallest value as IMIN, which are perpendicular to one another. The polar second moment of area (IP) reflects a long bones resistance to torsion and equals the sum of the maximum and minimum moments of area (IP = IMAX + IMIN).
Statistical analysis
Data were expressed in absolute terms and as a percentage of the nonplaying arm. Within each pubertal group, side-to-side differences were assessed using paired t-tests. ANOVA, with Tukey post hoc comparisons, was used to detect differences between pubertal groups. In the longitudinal analysis, subjects were divided according to pubertal status: prepuberty to prepuberty (n = 16), peripuberty to peripuberty (n = 15, includes prepuberty to peripuberty and peripuberty to peripuberty), and postpuberty to postpuberty (n = 16). Repeated measures ANOVA and analysis of covariance (ANCOVA) were used to determine changes over time in bone strength adjusted for bone size. Significance is reported as p < 0.05; borderline significances are reported at p < 0.1. All data are reported as mean ± SE unless otherwise stated.
RESULTS
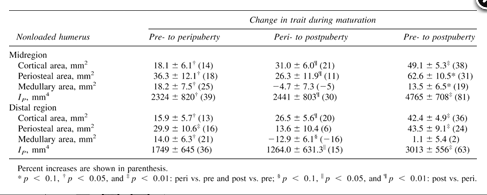
Growth itself, as reflected by structural changes in the nonloaded arm, resulted in a 14% increase in cortical area of the mid- and distal humerus from the pre- to peripubertal years because of greater periosteal expansion than medullary expansion (Table 2 and Fig. 2). Cortical area of the mid- and distal humerus were both ∼20% greater in the postpubertal players than in the peripubertal players (Table 2and Fig. 2). At the midhumerus, this was the result of periosteal expansion alone, whereas at the distal humerus, medullary contraction contributed to the larger cortical area.
Figure FIG. 2.. Schematic scaled representation of structural changes in the nonloaded arm. Cortical areas of the mid- and distal humerus were ∼14% greater in the peripubertal players than in the prepubertal players because of greater periosteal expansion than medullary expansion. Cortical areas of the mid- and distal humerus were ∼20% greater in the postpubertal players than in the peripubertal players. At the midhumerus, this was the result of periosteal expansion alone, whereas at the distal humerus, medullary contraction contributed to the larger cortical area.
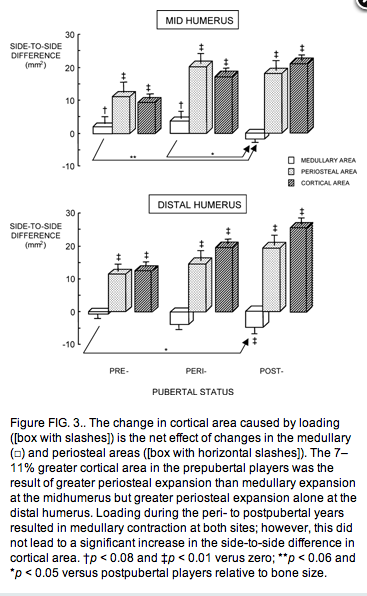
The effect of loading was reflected in the side-to-side trait differences. BMC and resistance to torsion (IP) of the humerus were 11–14% greater in the loaded arm than in the nonloaded arm in the prepubertal players (both p < 0.01) and did not increase further in peri- or postpubertal players despite longer duration of loading (Tables 1 and 3). The higher BMC was the result of a 7–11% greater cortical area in the prepubertal players, which was the result of greater periosteal expansion than medullary expansion at the midhumerus but greater periosteal expansion alone at the distal humerus (Table 3 and Fig. 3). Loading during the peri- to postpubertal years resulted in medullary contraction at both sites; however, this did not lead to a significant increase in the side-to-side difference in cortical area (Table 3 and Fig.3).
Figure FIG. 3.. The change in cortical area caused by loading ([box with slashes]) is the net effect of changes in the medullary (□) and periosteal areas ([box with horizontal slashes]). The 7–11% greater cortical area in the prepubertal players was the result of greater periosteal expansion than medullary expansion at the midhumerus but greater periosteal expansion alone at the distal humerus. Loading during the peri- to postpubertal years resulted in medullary contraction at both sites; however, this did not lead to a significant increase in the side-to-side difference in cortical area. †p < 0.08 and ‡p < 0.01 verus zero; **p < 0.06 and *p < 0.05 versus postpubertal players relative to bone size.
Similar observations were made in the 37 girls followed during the 12 months of follow-up. In particular, cortical area at the distal site increased 4% more in the loaded arm than in the nonloaded arm in the postpubertal players because of contraction of medullary area (2%,p < 0.05) and increased periosteal expansion (2%, NS).
DISCUSSION
Growth in the external size of a long bone, its cortical thickness, and the distribution of cortical bone about the neutral axis are determined by the absolute and relative behavior of the periosteal and endocortical bone surfaces along the length of the bone.(5, 6) Before puberty, periosteal apposition accounts for most of the increase in cortical area. Endocortical resorption creates an enlarging marrow cavity and partly offsets the increase in cortical area produced by periosteal apposition. The net result is an enlarged cortical area located further from the neutral axis, which has the effect of increasing its resistance to bending.(3) Late in puberty, periosteal apposition continues with a contribution from endocortical apposition(7); particularly at the distal humerus, where, in this study, the increase in cortical area was the result of equal contributions from periosteal and endocortical apposition.
In addition to surface specificity, growth is also region specific with more rapid maturation of distal regions than proximal regions. Distal segments of the appendicular skeleton mature before the proximal segments.(1,7,8) The longitudinal data indicate that when the subjects were older, endocortical contraction was detected at the midhumerus but not at the distal humerus.
Loading magnifies the structural changes produced during growth and this was detected by comparing the trait differences in the loaded and nonloaded arms. The data suggest that during growth the effect of exercise, like the effect of risk factors, is determined not only by the intensity of exercise or severity of illness, but also by the timing of exposure.(7, 9) Before puberty, loading magnified periosteal apposition. During the postpubertal period, loading magnified the effect of endocortical apposition, which makes an important contribution to cortical thickness in females. Indeed, endocortical apposition accounted for most of the greater side-to-side difference attained in the postpubertal years.
Most of the structural changes occurred early in the prepubertal years because adaptive changes in response to loading were sufficient to reduce the strains in bone that may lead to microdamage if not decreased.(10, 11) The only additional benefit achieved from tennis training later in puberty was contraction of the medullary cavity, which did not confer any additional increase in the structural rigidity of the bone. Similar effects have been reported in soccer players in whom increased duration of training beyond 6 h/week had no benefit on bone mass.(12) To further modify bone mass or architecture, other components of loading other than duration (i.e., magnitude or strain patterns) would have to increase, as reported in elite gymnasts.(13)
Heterogeneity in the response to loading has been reported in several studies.(14–17) The relative contributions of periosteal and endocortical modeling and remodeling varies along the whole length of a limb.(18) Local loading will modify each part of the geometry of the bone in accordance with the imposed load. In racquet sports, the greater humeral cortical area of the loaded versus the nonloaded arm is the result of both greater periosteal expansion and greater endocortical contraction; for instance, the relative contributions of periosteal expansion and endocortical contraction to the greater cortical thickness in the loaded arm than in the nonloaded arm in a study by Haapasalo et al. were 75:25 at the proximal humerus and 10:90 at both mid- and distal humerus.(15) In the study by Jones et al., the respective relative contributions of greater periosteal expansion and greater endosteal contraction to the greater cortical thickness were 60:40 in the anteroposterior dimension and 80:20 in the mediolateral dimension in male and female tennis players.(16)
Thus, loading affects both the periosteal and the endocortical surfaces but the magnitude of the effects vary according to whether the surface is anterior, posterior, medial, or lateral and according to whether the region is proximal, central, or distal along the bone’s length. Measurements of bone geometry in two dimensions using densitometry or X-rays cannot adequately describe this heterogeneity. Bone is not a cylinder with a circular perimeter and the assumption that loading will produce homogenous changes is flawed.
In conclusion, loading before puberty increases bone size and its resistance to bending. After puberty, loading increases the acquisition of bone on the endocortical surface with little benefit in the bone’s resistance to bending. Growth and the effects of loading were surface specific and varied along the length of the bone depending on the maturation of the region as well as the intensity and direction of loading. Increasing the bone’s resistance to bending and torsion is achieved by modifying the shape and mass of bone but not necessarily its density.
Acknowledgements
The authors thank radiographers Amanda Hunt and Glenn Rush for their technical assistance. They also thank the players and their parents for their time given to this study. This study was funded by grants from the Australian Research Council Grant and the School of Health Sciences, Deakin University.
REFERENCES
- 1. Garn S 1970 The Earlier Gain and Later Loss of Cortical Bone. Charles C Thomas, Springfield, IL, USA.
- 2. Ruff CB, Walker A, Trinkaus E 1994 Postcranial Robusticity in Homo. III: Ontogeny. Am J Phys Anthrop 93: 35–54.
- 3. Turner CH, Burr DB 1993 Basic biomechanical measurements of bone: A tutorial. Bone 14: 595–608.
- 4. Woodhead HJ, Kemp AF, Blimkie CJR, Briddy JN, Duncan CS, Thompson M, Lam A, Howman-Giles R, Cowell CT 2001Measurement of midfemoral shaft geometry: Repeatability and accuracy using magnetic resonance imaging and dual-energy X-ray absorptiometry. J Bone Miner Res 16: 2251–2259.
- 5. Seeman E 2002 An exercise in geometry. J Bone Miner Res 17: 373–380.
- 6. Seeman E 2001 Clinical review 137: Sexual dimorphism in skeletal size, density, and strength. J Clin Endocrinol Metab 86:4576–4584.
- 7. Bass S, Delmas PD, Pearce G, Hendrich E, Tabensky A, Seeman E 1999 The differing tempo of growth in bone size, mass and density in girls is region-specific. J Clin Invest 104: 795–804.
- 8. Preece MA, Hendrich I 1981 Mathematical modelling of individual growth curves. Br Med Bull 37: 247–252.
- 9. Seeman E, Karlsson M, Duan Y 2000 On exposure to anorexia nervosa, the temporal variation in axial and appendicular skeletal development predisposes to site-specific deficits in bone size and density: A cross-sectional study. J Bone Miner Res 15:2259–2265.
- 10. Frost H 1987 The mechanostat: A proposed pathogenic mechanism of osteoporosis and the bone mass effects of mechanical and nonmechanical agents. Bone Miner 2: 73–86.
- 11. Lanyon LE 1987 Functional strain in bone tissue as an objective and controlling stimulus for adaptive remodelling. J Biomech 20:1083–1093.
- 12. Karlsson MK, Magnusson H, Karlsson C, Seeman E 2001 The duration of exercise as a regulator of bone mass. Bone 28:128–132.
- 13. Bass S, Pearce G, Bradney M, Hendrick E, Delmas P, Harding A, Seeman E 1998 Exercise before puberty may confer residual benefits in bone density in adulthood: Studies in active prepubertal and retired female gymnasts. J Bone Miner Res 13: 500–507.
- 14. Haapasalo H, Sievanen H, Kannus P, Heinonen A, Oja P, Vuori I 1996 Dimensions and estimated mechanical characteristics of the humerus after long-term tennis loading. J Bone Miner Res 11: 864–872.
- 15. Haapasalo H, Kontulainen S, Sievanen H, Kannus P, Jarvinen M, Vuori I 2000 Exercise-induced bone gain is due to enlargement in bone size without a change in volumetric bone density: A peripheral quantitative computed tomography study of the upper arms of male tennis players. Bone 27: 351–357.
- 16. Jones HH, Priest JD, Hayes WC, Tichenor CC, Nagel DA 1977 Humeral hypertrophy in response to exercise. J Bone Joint Surg Am 59A: 204–208.
- 17. Huddleston A, Rockwell D, Kulund DN, Harrison B 1980 Bone mass in lifetime tennis athletes. JAMA 244: 1107–1109.
- 18. Hsieh YF, Robling AG, Ambrosius WT, Burr DB, Turner CH 2001 Mechanical loading of diaphyseal bone in vivo: The strain threshold for an osteogenic response varies with location. J Bone Miner Res 16: 2291–2297.
From an old link on P. Zhangs’s article on joint loading on hindlegs of pre-pubescent mice HERE…. For the Full Text of the study click HERE.
Lengthening of mouse hindlimbs with joint loading.
Source
Department of Biomedical Engineering, Indiana University-Purdue University Indianapolis, Indianapolis, IN 46202, USA.
Abstract
For devising clinical approaches to treating limb length discrepancies, strategies that will generate differential longitudinal growth need to be improved. This report addresses the following question: does knee loading increase bone length of the loaded hindlimb? Knee loading has been shown to induce anabolic responses on the periosteal and endosteal surfaces, but its effects on longitudinal bone growth have not yet been examined. In the present studies, loads were applied to the left hindlimb (5-min bouts at 0.5 N) of C57/BL/6 mice (21 mice, ~8 weeks old). Compared to the contralateral and age-matched control groups, knee loading increased the length of the femur by 2.3 and 3.5%, together with the tibia by 2.3 and 3.7% (all P < 0.001), respectively. In accordance with the length measurements, knee loading elevated BMD and BMC in both the femur and the tibia. Histological analysis of the proximal tibia revealed that the loaded growth plate elevated its height by 19.5% (P < 0.001) and the cross-sectional area by 30.7% (P < 0.05). Particularly in the hypertrophic zone, knee loading increased the number of chondrocytes (P < 0.01) as well as their cellular height (P < 0.001) along the length of the tibia. Taken together, this study demonstrates for the first time the potential effectiveness of knee loading in adjusting limb length discrepancy.
- PMID: 19890688 [PubMed – indexed for MEDLINE]