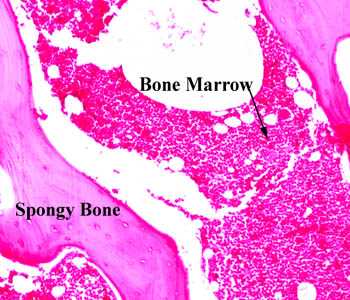
The White spots within bone marrow are adipose tissue. There are four above the arrow for instance.
Bone size and bone strength are increased in obese male adolescents.
“We recruited 51 male ObAs (10-19 years) at the entry of a residential weight-loss program and 51 healthy age-matched and 51 bone-age-matched controls. vBMD and geometric bone parameters, as well as muscle and fat area were studied at the forearm and lower leg by peripheral quantitative computed tomography. Muscle force was studied by jumping mechanography. In addition to an advanced bone maturation, differences in trabecular bone parameters (higher vBMD and larger trabecular area) and cortical bone geometry (larger cortical area and periosteal and endosteal circumference) were observed in ObAs both at the radius and tibia at different pubertal stages. After matching for bone age, all differences at the tibia, but only the difference in trabecular vBMD at the radius, remained significant. Larger muscle area and higher maximal force were found in ObAs compared with controls, as well as higher circulating free estrogen, but similar free testosterone and IGF-I levels. ObAs have larger and stronger bones at both the forearm and lower leg. The observed differences in bone parameters can be explained by a combination of advanced bone maturation, higher estrogen exposure, and greater mechanical loading resulting from a higher muscle mass and strength. ”
Obese individuals have a higher bone age than non-obese individuals until age 16. Obese individuals had taller height than age matched controls but shorter height than bone age matched controls.
“higher values of trabecular vBMD, trabecular area, periosteal circumference, and cortical area at the different pubertal stages in the obese group. ” So bodyfat causes increase in bone parameters outside of the growth plate. This includes the bone age matched group and not just the age matched group.
Obese individuals had increased estrogen and leptin but similar levels of free testosterone.
What would be interesting if the fat itself did not increase various hormones and genes to cause bone growth or some kind of loading effect. But if the fat within the bone itself caused an expansion of bone parameters.
The main difference between a growth plate and adipose tissue is that adipose tissue is disorganized as you can see in the image of the bone marrow however adipose tissue cells are huge. So is it possible that there could be enough adipose tissue cells to cause an expansion of the bone even if they are not coordinated like a growth plate.
The effect of weight on the femur: a cross-sectional analysis.
“if stresses associated with biomechanical modifications of the obese surpass the strain threshold of a bone or bony location, it is possible that discernible differences in long-bone morphology could be observed between different weight categories as a direct result of long-term, abnormal mechanical compensation.”
“The Pearson’s product-moment correlation coefficient results show no correlation between weight and stature. “<-Since only very large amounts of weight would influence stature it’s possible that effect of extreme weights are overlooked.
Mediolateral dimensions of the bone at the midshaft at the bone increased at 4 out of 5 of the sites measured in the bone. It’s possible that other parameters were increased but not statistically significant. Anteriorposterior dimensions were increased only at the midshaft.
Is it the adipose tissue cells themselves that increase the bone dimensions or is it a weight loading effect increasing the dimenions. The question is why would the bone increase in size in only one dimension. The increase in bone size being mainly in one axis is consistent with it being a weight loaded effect and not a result of internal forces from adipose tissue cells.
“research has shown elongation of the proximal ML dimension of the femur in pregnant women”
“As ML diameter measures resistance to ML bending, these results suggest that as weight increases, alterations to the femoral angle result in greater ML pressures, forcing the femur to adapt or risk failure.”
Reduced size-independent mechanical properties of cortical bone in high-fat diet-induced obesity.
” femora from C57BL/6 mice fed either a HFD or standard laboratory chow (Chow) were evaluated for structural changes and tested for bending strength, bending stiffness and fracture toughness. Here, we find that in young, obese, high-fat fed mice, all geometric parameters of the femoral bone, except length, are increased, but strength, bending stiffness, and fracture toughness are all reduced. This increased bone size and reduced size-independent mechanical properties suggests that obesity leads to a general reduction in bone quality despite an increase in bone quantity; yield and maximum loads, however, remained unchanged, suggesting compensatory mechanisms. We conclude that diet-induced obesity increases bone size and reduces size-independent mechanical properties of cortical bone in mice.”
Mice were fed high fat diet over 19 weeks. 4 week old mice were used.
“the HFD group showed a 34% increase in serum IGF-I concentration compared to Chow”
Cellular dynamics and tissue interactions of the dura mater during head development
“Morphogenesis of the cranial bones and sutures is dependent on tissue interactions with the dura mater, which control the size and shape of bones as well as sutural patency. Development of the brain also involves interactions with dura mater: secretion of stromal derived factor 1 (SDF-1) is a critical event in directing migration of the external granular layer precursors of the cerebellar cortex and the Cajal-Retzius (CR) cells of the cerebral cortex. The dura mater is also required for growth of the hippocampal dentate gyrus. Wnt1Cre/R26R transgenic reporter mice were used to study the origin and fates of the cells of dura mater during head development. The dura mater of mammals is derived entirely from the cranial neural crest. Beginning around neonatal day 10 (N 10), the dura mater is infiltrated by cells derived from paraxial mesoderm, which later come to predominate. Over the course of infancy, the neural crest–derived cells of the dura mater become sequestered in niche-like distribution characteristic of stem cells. Simultaneously, dura mater cells underlying the sagittal suture migrate upward into the mesodermally-derived mesenchyme separating the parietal bones. Although initially the parietal bones are formed entirely from paraxial mesoderm, the cellular composition gradually becomes chimeric and is populated mainly by neural crest–derived cells by N 30. This occurs as a consequence of osteoblastic differentiation at the dura mater interface and intravasation of neural crest–derived osteoclastic and other hematopoietic precursors. The isolated cells of the dura mater are multipotent in vitro, giving rise to osteoblasts, neuronal cells and other derivatives characteristic of cranial neural crest, possibly reflecting the multipotent nature of dura mater cells in vivo.
” neural crest cells can be found throughout the intrafrontal suture. These cells give rise to fibroblast-like mesenchymal cells in the sutures, as well as chondrocytes, osteoblasts, and osteocytes in developing bones.”
“Mineralized bone is incapable of interstitial growth{this would explain why adipose tissue cells don’t cause interstitial growth, however is unmineralized bone capable of interstitial growth; unfortunately there is no citation}, and bones grow at the marginal growth sites—growth plates in long bones and sutures in the skull. ” I also couldn’t find any emails either so I can’t ask where they retrieved that conclusion from.
Further research shows that the possibility of interstitial growth is related to the the rigidity of the ECM. So adipocytes may be capable of interstitial growth if the ECM is too rigid.
I write about the optimal stiffness of ECM for chondrocyte differentiation here. However, the stiffness for ECM for chondrocyte differentiation may be different from that for interstital growth. Here I mention, that the compounds that give the bone ECM it’s stiffness are Calcium, Phosphorus, and Vitmain D. However, people with deficiencies in those three compounds do not grow taller. Also, mentioned is that demineralized bone matrix is an effective scaffold for chondroinduction.
In conclusion, the reason that adipose cells do not cause interstitial growth in bone despite being enormous and potentially present in massive quantities is that the ECM of bone is too stiff due to the mineral content. Although during longitudinal growth, the bone is stiff at the bony area between the top and bottom area of the growth plate. Thus, a key factor for micro-growth plate success via induction by LSJL is to reduce bone ECM stiffness. LSJL may do this itself by causing interstitial fluid flow and shear strain.
LSJL dincreasing interstitial fluid flow and shear strain can be supported by the histological slides presented here. The pink area which represents the bone appears to be much less rigid(compare slides A and B).

Maybe having a band around the emphasis over night will promote upwards growth by controlling bone shape slightly and increasing pressure slightly, what do you think?
Very good,
Micael what do you think of researching more about the growth of vertebrae in spine?
Lsjl, Lipus or Pemf could be performed on spine the only thing that worries me is that it could grow inwards theirs start to touch a nerve
that is actually very legitimate. However any technique we propose has to extremely careful since paralysis is a potential result.
Pingback: Manipulating ECM stiffness for height increase | Natural Height Growth
Pingback: Height Increase Techniques that are unlikely to work - Natural Height Growth