Bmpr1a is downregulated by aging in bone marrow. LSJL upregulates Bmpr1b but expression of Bmpr1a is modulated by normal mechanical loading. BMP-2 interacts with Bmpr1a. LSJL upregulates BMP-2. Cells in the perichondrial groove express Bmpr1a. Persistent upregulation of Hoxa2 which causes short stature downregulates Bmpr1a. Bmpr1a is upregulated in a fractured tibia. These facts are consistent with the observation that bmpr1a could play an on/off-switch in neo-growth plate formation.
BMP Receptor 1A Determines the Cell Fate of the Postnatal Growth Plate.
“Embryos deficient in Bmp receptor (Bmpr)1a or Bmpr1b in cartilage display subtle skeletal defects; however, double mutant embryos develop severe skeletal defects, suggesting a functional redundancy that is essential for early chondrogenesis. In this study, we examined the postnatal role of Bmpr1a in cartilage. In the Bmpr1a conditional knockout (cKO, a cross between Bmpr1a flox and aggrecan-CreER (T2) induced by a one-time-tamoxifen injection at birth and harvested at ages of 2, 4, 8 and 20 weeks), there was essentially no long bone growth with little expression of cartilage markers such as SOX9, IHH and glycoproteins. Unexpectedly, the null growth plate was replaced by bone-like tissues, supporting the notions that the progenitor cells in the growth plate, which normally form cartilage, can form other tissues such as bone and fibrous; and that BMPR1A determines the cell fate. ”
“During endochondral ossification, the progenitor cells committed act as the stem cells that replenish the pool of proliferative chondrocytes in the resting zone of the growth plate. The resulting daughter cells exit the cycle and undergo maturation via prehypertrophic, hypertrophic and terminal hypertrophic processes, followed by calcified cartilage formation. The vascular invasion then leads to the removal of cartilage, formation of bone marrow, and new bone formation and growth.”
“Is [there] a molecule that initiates and guides the progenitor cell in the resting zone of the growth plate, which solely differentiates chondrogenesis?”<-We want to find this molecule and induce it in adult bone.
” The failure of long bone growth indicates that there is no new progenitor cells added after deletions of Bmpr1a gene by one time injection of tamoxifen at newborn stage.”<-This is bad news that there may be a fixed pool of progenitor cells that is used up. However, other stem cells could become progenitor cells after the right mechanical stimulus.

“The work with a one-time injection of tamoxifen to remove Bmpr1a in cartilage at birth, which leads to a lack of long bone growth postnatally, raises the possibility that the progenitor cell number might already be present in the growth plate at birth with no more new progenitor cells added. This observation may explain why the rodent growth plate remains unfused during the animal’s lifetime but there is essentially no long bone lengthening after 28-30 weeks. In fact, in the 5-month-old control mouse, there is very limited cell proliferation in the growth plate compared to the early stage ”
So the goal in a bone lengthening program is to alter cell morphology of stem cells to be more like the progenitor cells present in the zone of ranvier and then to upregulate bmpr1a expression to encourage a chondrogenic fate.
This fixed pool of progenitor cells is also supported by s1p-ko mice which can form new growth plates from this pool of progenitors.
IGF-2 could play a role in priming MSCs to be more like the progenitor cells that form new growth plates. IGF-2 has been used to induce longitudinal bone growth. The LSJL scientists have also suggested that IGF-2 injections may be beneficial to LSJL lengthening. CTGF is linked to IGF2 and is another target to increase for supplements as well as HMGA2. IGF2 is downregulated almost 1000-fold during senescence which further links IGF2 to the creation of progenitor cells.
I couldn’t find any supplements that increase IGF-2. There’s a supplement called IGF-2 but that just seems to be the brand name.
Epigenetic consequences of a changing human diet.
“The ultimate methyl donor for epigenetic-methylation reactions is S-adenosylmethionine that is produced by the methylation cycle and it has been reported that periconceptional folic acid use alters the level of methylation within IGF2. A larger study of human pregnancy also observed an effect of folic acid use on IGF2 methylation in the offspring but the effect was restricted to folic acid use after 12 weeks gestation when women are not recommended to take the supplement. Late gestation use of folic acid was also associated with reduced LINE-1 methylation and altered paternally expressed gene 3 (PEG3) methylation. Three of the four significant associations with folic acid use and folate status were negative and one was positive, suggesting that it may be naive to assume that this is a simple substrate limitation effect or that the supply of nutrients involved in the methylation cycle will affect all genes equally.
Imprinting occurs before fertilisation but changes in imprinting methylation in animal models in response to nutritional exposures have been demonstrated into the early post-natal period for IGF2, after which the imprint is apparently fixed”
Choline can result in hypermethylation of IGF2 in the liver. Liver production of IGF2 is not stimulated by GH in adult mammals. In adult mammals, IGF2 does not seem to respond to physiological status or vary with growth rate.
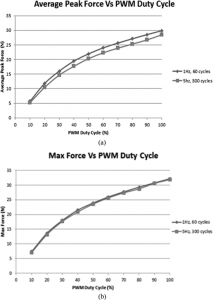
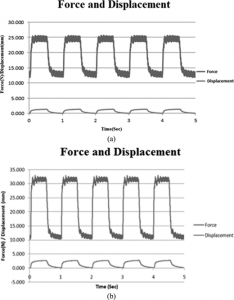
“In this study, a single period of cyclic mechanical stretch (0.5 Hz, 2,000 microepsilon) [for 40 minutes] was performed on rat bone marrow MSCs. Cellular proliferation and alkaline phosphatase (ALP) activity was examined. The mRNA expression of six bone-related genes (Ets-1, bFGF, IGF-II, TGF-beta, Cbfa1 and ALP) was detected using real-time quantitative RT-PCR.
The results showed that mechanical strain can promote MSCs proliferation, increase ALP activity, and up-regulate the expression of these genes. A significant increase in Ets-1 expression was detected immediately after mechanical stimulation, but Cbfa1 expression became elevated later. The temporal expression pattern of ALP coincided perfectly with Cbfa1.
The results of this study suggest that mechanical strain may act as a stimulator to induce differentiation of MSCs into osteoblasts{but could be chondrocytes with increase in bmpr1a levels}.”
This was the load applied to the cells during the study it’s reasonable to expect that the load is similar to that which is applied by LSJL.
“The transcription profiles of IGF-Ⅱand TGF-β were similar. Both genes reached maximum transcription immediately after mechanical strain and mRNA levels then decreased with time, except for a slight increase at 6 hours.”
The age of rats is not given and is vital information given that IGF2 is an age related gene.
It’s conceivable that LSJL modulates Bmpr1a expression given the study that shown it can be modified by mechanical loading and that LSJL modulates IGF2 expression given the study above that showed that it was upregulated by mechanical loading.
But still finding supplements that upregulate IGF2 and Bmpr1a would be exceptionally helpful.