Fzd9 is upregulated in cartilage formation.
Since fracture healing often involves endochondral ossification, similar processes as those involved in fracture healing could induce height growth in intact bone. As for whether a gap is needed for longitudinal bone growth remember that bony bridging can be overcome. And it may be the limited pool of progenitor cells that limit growth and not the prescence or absence of the gap. S1p-ko mice are capable of forming new growth plates from this progenitor cell pool.
Fzd9’s role in height increase seems to be later and relates to the conversion of the cartilage to bone. So Fzd9 will affect height increase per chondrocyte but will not create chondrocytes in the first place.
The below study does provide us with the insight that the periosteum is the location of the key region of progenitor cells that can undergo growth plate chondrogenesis and increase height. How do we get these stem cells past the cortical bone and into the bone itself where they have potential to increase height?
The wnt serpentine receptor frizzled-9 regulates new bone formation in fracture healing.
“The serpentine receptor Fzd9, a Wnt receptor of the Frizzled family, is essential for osteoblast function and positively regulates bone remodeling via the non-canonical Wnt pathway without involving β-catenin-dependent signaling. Here we investigated whether the Fzd9 receptor is essential for fracture healing using a femur osteotomy model in Fzd9 (-/-) mice. After 10, 24 and 32 days the fracture calli were analyzed. Our results demonstrated significantly reduced amounts of newly formed bone at all investigated healing time points in the absence of Fzd9 and, accordingly, a decreased mechanical competence of the callus tissue in the late phase of fracture healing. In contrast, cartilage formation and numbers of osteoclasts degrading mineralized matrix were unaltered{So could Fzd9 not be linked to growth plate formation?}. Canonical Wnt-signaling was not affected in the absence of Fzd9 in osteoblasts as well as in proliferating and mature chondrocytes within the fracture callus. The expression of established differentiation markers was not altered in the absence of Fzd9, whereas chemokines Ccl2 and Cxcl5 seemed to be reduced. Collectively, our results suggest that non-canonical signaling via the Fzd9 receptor positively regulates intramembranous and endochondral bone formation during fracture healing, whereas it does not participate in the formation of cartilage or in the osteoclastic degradation of mineralized matrix.”
“Wnt4, Wnt5a, Wnt10b, Wnt11 and Wnt13, Wnt receptors, including Fzd1, 2, 4 and 5, the co-receptors Lrp5 and Lrp6, β-catenin and Wnt target genes, including Runx2, a transcription factor associated with osteoblast differentiation, were up-regulated in the facture callus during bone regeneration”
“FZD9 is one of the genes, whose homozygous deletion in humans induces Williams-Beuren syndrome, a disorder associated with multiple manifestations, including low bone mass”
According to Statural growth in Williams-Beuren syndrome, Williams-Beuren syndrome does reduce height.
“After 10 days, most of the callus was composed of cartilage and fibrous tissue. New bone formation, which started at some distance from the fracture gap near the periosteum, was significantly reduced by 45% in the absence of Fzd9, whereas the amount of cartilage was not significantly affected”<-So the periosteum is where the pool of chondrogenic progenitor cells resides and Fzd9 does not appear to affect to conversion of these progenitor cells to chondrocytes.
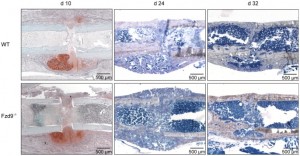 You can almost see the stem cells flooding into the fracture gap of the bone. Note that this region of stem cells seems to extend slightly beyond the region of the fracture gap in d24 and d32.
You can almost see the stem cells flooding into the fracture gap of the bone. Note that this region of stem cells seems to extend slightly beyond the region of the fracture gap in d24 and d32.
“bone marrow stromal cells derived from Fzd9-knockout mice exhibited a diminished capacity to proliferate and form mineralized matrix”
