Osteocyte-secreted IGF-1 may manipulate height growth via IGFBP secretions but this would only have an effect on adult individuals if IGFBPs could induce chondrogenesis on their own.
“Osteocytes secrete large amounts of insulin-like growth factor (IGF)-I in bone.”
“a regulatory role for osteocyte-derived IGF-I in the osteogenic response to mechanical loading”
“transgenic mice with ablation[removal] of osteocytes were unresponsive to unloading and had an impaired mechanotransduction”<-For more on this study see below.
“The long bones of transgenic mice with overexpression of IGF-I in bone showed enhanced osteogenic response to in vivo mechanical loading.”<-thus release of IGF-1 by osteocytes could be a key mediator of loading on bone shape.
“Conditional disruption of Igf1 gene in osteocytes blocked the loading-induced expression of early mechanoresponsive genes, i.e., cyclooxygenase-2 (Cox2), Igf1, and c-Fos”
“ince conditional deletion of Igf1 gene in hepatic cells, which reduced circulating IGF-I levels by >75%, had no effects on bone length and size, but targeted disruption of Igf1 gene in mature osteoblasts or chondrocytes greatly reduced bone length and size without affecting the circulating IGF-I level, it appears that locally produced bone-derived IGF-I, and not the circulating liver-derived IGF-I, is essential for the developmental bone growth.”
“the osteocytes-derived IGF-I-dependent regulation of longitudinal bone growth may involve osteocyte-derived soluble factors. A potential candidate is the IGF binding proteins (IGFBPs). IGF-I has paracrine effects on bone cell production of IGFBPs, and many IGFBPs have IGF-dependent and -independent actions on bone turnover. Changes in Igf1 expression in a number of cell types have been associated with alterations in the IGFBPs expression profile. For example, target disruption of Igf1 in chondrocytes reduced IGFBP5 expression in the growth plate cartilage. Conditional disruption of Igf1 in mature osteoblasts decreased bone levels of IGFBP3 and IGFBP4. Conversely, conditional disruption of Igf1 in osteocytes increased plasma IGFBP3 level and decreased plasma IGFBP5 level, raising the intriguing possibility that the reduced bone production of the stimulatory IGFBP5 and the increased bone production of the inhibitory IGFBP3 in osteocyte conditional KO mutants may in part contribute to the reduced longitudinal bone growth.”
“The osteocyte Igf1 conditional KO mice [has] 8-12% shorter bone length and small bone size”
Since part of the LSJL hypothesis is that LSJL has a greater effect than normal. Let’s examine the study which found that removal of osteocytes dapened mechanical loading.
Targeted Ablation of Osteocytes Induces Osteoporosis with Defective Mechanotransduction
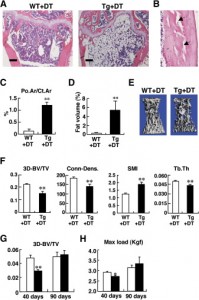
“Following a single injection of DT, approximately 70%–80% of the osteocytes, but apparently no osteoblasts, were killed. Osteocyte-ablated mice exhibited fragile bone with intracortical porosity and microfractures, osteoblastic dysfunction, and trabecular bone loss with microstructural deterioration and adipose tissue proliferation in the marrow space, all of which are hallmarks of the aging skeleton. Strikingly, these “osteocyte-less” mice were resistant to unloading-induced bone loss”
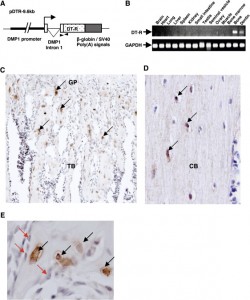
Here’s an image that shows the impact of osteocyte ablation on mouse growth plates:
 Tg+Dt A shows the growth plate of mouse with less osteocytes. You can see that it is more disorganized but whether it affects longitudinal bone growth is unclear. In E is an image of a vertebrae and the ablated osteocyte bone may be shorter by eyeballing it.
Tg+Dt A shows the growth plate of mouse with less osteocytes. You can see that it is more disorganized but whether it affects longitudinal bone growth is unclear. In E is an image of a vertebrae and the ablated osteocyte bone may be shorter by eyeballing it.
Here’s an image of the scattered growth plate in the ablated osteocyte bone:
So, osteocyte IGF-1 contributes to longitudinal bone growth at least by increased organization. Since increased organization was not apparent in LSJL growth plates, it is still likely that LSJL can stimulate height by a method not available via typical mechanical loading.