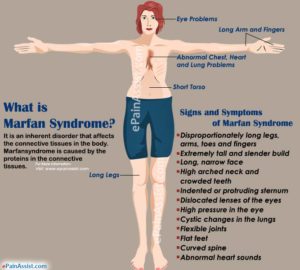
If we know why people with Marfan’s stature grow taller we can incorporate that into our quest to grow taller.
It’s interesting to note that all the things that all the things that grow taller with Marfan’s have more joint mobility especially the fingers and toes. What doesn’t grow with Marfan’s doesn’t have as much joint mobility such as the torso. So Marfan’s syndrome creating additional joint spacing via looser ligaments could be key to growth.
The Fibrillin Microfibril Scaffold: A Niche for Growth Factors And Mechanosensation?
“The fibrillins, large extracellular matrix molecules, polymerize to form “microfibrils.” Fibrillin microfibril scaffold is populated by microfibril-associated proteins and by growth factors, which are likely to be latent{So more microfibrils may catch more growth factors helping people grow taller?}. The scaffold, associated proteins, and bound growth factors, together with cellular receptors that can sense the microfibril matrix, constitute the fibrillin microenvironment. Activation of TGFβ signaling is associated with the Marfan syndrome, which is caused by mutations in fibrillin-1. Mutations in fibrillin-1 cause the Marfan syndrome as well as Weill-Marchesani syndrome (and other acromelic dysplasias) and result in opposite clinical phenotypes: tall or short stature; arachnodactyly or brachydactyly; joint hypermobility or stiff joints; hypomuscularity or hypermuscularity. These different syndromes are associated with different structural abnormalities in the fibrillin microfibril scaffold and perhaps with specific cellular receptors (mechanosensors). How does the microenvironment, framed by the microfibril scaffold and populated by latent growth factors, work? Fibrillin microfibril niche [is] a contextual environment for growth factor signaling and potentially for mechanosensation.”
“fibrillins and LTBPs may bind and sequester different members of the TGFβ superfamily of growth factors.”
“Disruption of the fibrillin microfibril scaffold was implicated in the Marfan syndrome, and mutations in the gene for fibrillin-1 (FBN1) were shown to cause Marfan syndrome. Mutations in the gene for fibrillin-2 (FBN2) were found in congenital contractural arachnodactyly (now called Distal Arthrogryposis, Type 9 or DA9)”
“fibrillin-1 deficiency [may result] in abnormal activation of TGFβ signaling”
” fibrillins interact with the BMP-7 complex”
“TGFβ propeptides bind covalently to an 8-cysteine domain in LTBPs”
“TGFβ should be regarded as a “cellular switch”, and “its true function is to provide a mechanism for coupling a cell to its environment””
“Bound to the fibrillin microfibril scaffold, the large latent TGFβ complex, as well as BMP prodomain/growth factor complexes, require activation to initiate growth factor signaling. For activation of TGFβ, integrin binding to the propeptide might change the conformation of the propeptide such that the growth factor is allowed to interact with its receptors”
“If binding to fibrillin alters the conformation of the BMP prodomain such that its growth factor is not accessible to BMP receptors, mechanisms of activation will also be required for BMP signaling.”
“With the possibility of multiple interactions between growth factors and the fibrillin microfibril scaffold, tissue-specific microenvironments can be achieved through regulation of growth factor gene expression.”