Since the new proposed LSJL modality, involves loading sites where bones attach to each other specifically at the enthesis, due to the similarity of the enthesis of the zone of ranvier which is where the pool of stem cells resides to help formulate the growth plate.
Here’s some images and quotations from the orthopaedics blog:
Note although this article mainly refers to tendon enthesis, a majority of the info should apply to ligament enthesis(which tend to be attached closer to the regions near where the growth plate used to be).
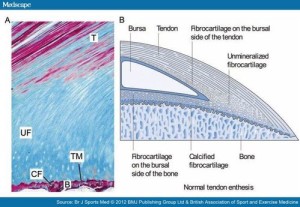
This next image shows in much more detail into how the enthesis really integrates into the bone and how enthesis stem cells could be stimulated to form a mini growth plate. Bone erosion could occur allowing this growth plate to extend across the bone. In fact, in arthritis which is associated with thickening of the enthesis’ bone erosion does occur.:
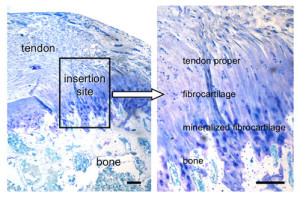
Note how the mineralized fibrocartilage integrates into the bone. The thickening of the enthesis and the erosion of bone in some forms of arthritis, I could only find citations of it occuring in tendons and have not found instances of it resulting in increased length. But that is likely a result of tendons being more subject to mechanical loading and this new method of LSJL being a novel way to mechanical load the ligaments. Also ligaments in contrast to tendons are in a better position to contribute to longitudinal bone growth. In fact, most of the studies I could find on mechanical loading on ligaments referred to loading on the ligament cells themselves rather than loading of the ligaments in the body.
Ligament injuries tend to occur due to heavy impact or overstretching of the ligament. Pressing of the bones against each other is not a common way of ligament injury indicating that this method of LSJL loading is in fact a novel way of loading the ligaments.
“There is physiological thickening of the fibrocartilage with stress.”<-This tends to happen more with tendons as they are attached to muscle but with LSJL we can encourage it to happen at the ligament fibrocartilage.
” there are other components adjacent to the enthesis proper which also share the stress forces and are termed the “Enthesis organ”. These include the Periosteal fibrocartilage, the Sesamoid fibrocartilage, the Fat pad and Bursa. The Synovial entheseal complex is a concept that the adjacent bursa or joint lining share stress forces, especially compressive forces and are an integral part of the enthesis organ”<-These will be different for the ligament.
Here’s some info from another site that could be pertinent:
Bone Erosion at Normal Insertions
“Bone erosion is a process whereby the surface of a bone (the bone cortex) is degraded or eroded and is most typically seen in the setting of inflammation. However, the normal skeleton appears to be riddled with microscopic erosions. The enthesis is a highly mechanically stressed site which leads to microtrauma to the immediately adjacent bone. This is the basis for small erosions in the normal non-diseased skeleton which likely repair spontaneously.”<-Can we cause sufficient bone erosion as to allow for a new growth plate?
“The early phases of erosion may start to damage or loss of the shock absorbing fibrocartilage that covers the bone. “<-We don’t want this however as this would be the foundation of the neo growth plate.
“Normal small joints tend to develop microscopic erosions at sites where the ligament immediately adjacent to the enthesis compresses the bone. This is because the shape of the bone leads to the forces being spread over a wide area that contributes to damage. This occurs at a structure termed a synovio-entheseal complex.”
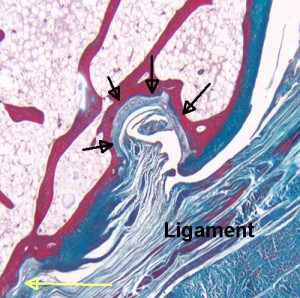
“The black arrows show a microscopic erosion over a knuckle joint. The overlying ligament is shown. The yellow arrow shows the point of ligament attachment closest to the joint cavity. Small blood vessels in the base of the erosion are likely linked to attempted repair. ”
“Sometimes the bone compression by the enthesis organ transmits stresses to the underlying bone and this initially manifests as a small cyst. Later on the roof of this may cave in leading to erosion. ”
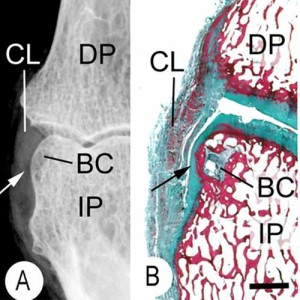
“This is an X-ray (A) and a corresponding tissue section (B). It shows a small bone cyst (BC). This is underneath the cartilage lining the side of the bone. (black arrow). Damage occurs here because the ligament (CL) presses against the bone as it runs between the joints. “<-We’d need more information about the bone cyst to see how promising it is in terms of neo growth plate formation.
Here’s another paper about the enthesis:
Bone Surface Micro-Topography at Craniofacial Entheses: Insights on Osteogenic Adaptation at Muscle Insertions
“Macroscopic details of the bone–muscle interface are represented by a mosaic of calcified features inclusive of fossae, tuberosities, crests, and ridges. These features are in part of an adaptive osteogenic response to dissipate forces of localized mechanical loading{how much can they be influenced by mechanical loading?}. In an osteoarchaeological or paleontological context, these features are interpreted as “musculoskeletal stress markers” to infer habitual behaviors. Microscopic surveys of bone surface topography of the enthesis can reveal localized osteogenic topologies. These features illustrate the developmental mechanisms that produce these bony forms and contribute to an evidential basis to read these structures. Microscopic osteogenic topographies at sites of gnathic muscle attachments located in the craniofacial skeleton were explored in reference to extrapolated loading vectors in an ontogenetic series of craniofacial skeletons of the primate (Procolobus verus). Epoxy resin replicas of bone surfaces were made, and micro-topographical detail viewed with Scanning Electron Microscope. Osteoclastic bone remodeling was found at entheses associated with presumptive net tensile loading. Mineralized fibrocartilage was present at entheses, associated with presumptive net compressive loading{which would be more beneficial for height growth mineralized fibrocartilage or osteoclastic bone remodeling? I’d say osteoclastic bone remodeling because bone is not capable of interstitial growth and by allowing for osteoclastic bone remodeling we allow for tissues that it is}. Collectively, these outcomes suggest that entheses develop through adaptive osteogenic activity in response to differential vectors of local mechanical loading. However, the presence of mineralized fibrocartilage also suggests that proliferative cartilage has a role in the development of bone eminences providing functional processes. This study concludes that the vector of muscle loading at entheses as well as proliferative fibrocartilage is influencing the form of bony eminences in the primate craniofacial skeleton defining functional and species defining morphologies.”
“The majority are considered to be of the fibrocartilaginous type where zones of uncalcified and calcified fibrocartilage are interposed between the dense fibrous tissue of the tendon and a bone matrix”<-since they are intermixed stimuli that affects bone should benefit the enthesis like
interstitial fluid flow.
“The bone-penetrating extrinsic fibers known as Sharpey’s fibers are considered as the defining feature when these fibrous tendons are directly anchored to the bone”
“There are emerging reports that contradict the above assertion, for example, zones of mineralized and unmineralized fibrocartilage have been identified at the deltoid insertion into the diaphysis of the humerus. This fibrocartilaginous zone can act as a mini growth plate to produce mass for the deltoid tuberosity. Furthermore, these cells are likely to be derived from a different source to those that form long bone analgen as they express the transcription factor Scleraxis (Scx) consistent with a syndetome origin, the precursors for tendon cells”
” muscle action of this enthesis aligned with extrapolated vectors of net tension at this locality result in osteoclastic activity”
Above is the osteoclastic activity mentioned.
“These cellular based osteological functions can produce gross shape change of bone processes through micro anatomical bone surface modeling and remodeling modulated by the loading vector“<-so the enthesis can alter the bone this is huge!
“Loads extrapolated to be perpendicular to bony substrate exhibited osteogenic responses that adaptively opposed the loading vector. When in net tension, osteoclastic remodeling topographies were observed contributing to the formation of fovea and depressions on the bone surface. These forms redistribute the loading vectors to the periphery and increase the surface area of attachment. This differs from entheses that are subject to net compression where topographies notably consistent with mineralized fibrocartilage. In these regions, gross bony eminences and processes form that provide mechanical leverage to muscle contraction.”<-by manipulating compression and tension how can we manipulate the bone?
Above image is the direct impact of the enthesis on mandible shape.
“Schematic of bone form changes in the mandibular ramus associated with masticatory muscle enthesis. The inner tracing depicting infant mandibular outline followed by adolescent to adult (outside tracing) depicting hypothesized growth trajectories developed from surface micro topographies indicative of differential osteogenic activity. The large arrows depicting the growth vector at the angle, coronoid, and condyle of the mandible. Bone mass facilitated by mineralized fibrocartilage deposits indicated by (0) at sites of net compression. Subtle shape changes to the condylar neck associated with remodeling indicated by minus sign (−) facilitated migratory pathway consistent with the appositional growth vector of the condyle. Where the masseter muscle belly lies on the ascending ramus a remodeling field can be observed indicated by minus (−). The ramus of the mandible in particular is modulated by the muscle anatomical units where intrinsic and extrinsic modulated proliferative fibrocartilage associated with entheses establishes species and functional defining forms.”
” locations of these chondron dense fields are found perpendicular to the growth and coincident with load vectors. This provides an avenue for ossification and progressive bony growth at these muscle attachments where the entombed fiber become destined to become fields of the bone penetrating Sharpey fiber”
“Entheses transmit force from tendons and ligaments to the skeleton. Regional organization of enthesis extracellular matrix (ECM) generates differences in stiffness required for force transmission. Two key transcription factors co-expressed in entheseal tenocytes, scleraxis (Scx) and Sox9, directly control production of enthesis ECM components.{perhaps gene therapy of Scx and Sox9 could be used to stimulate the enthesis and enhance limb lengthening recovery} Formation of embryonic craniofacial entheses in zebrafish coincides with onset of jaw movements, possibly in response to the force of muscle contraction. We show dynamic changes in scxa and sox9a mRNA levels in subsets of entheseal tenocytes that correlate with their roles in force transmission. We also show that transcription of a direct target of Scxa, Col1a, in enthesis ECM is regulated by the ratio of scxa to sox9a expression. Eliminating muscle contraction by paralyzing embryos during early stages of musculoskeletal differentiation alters relative levels of scxa and sox9a in entheses, primarily owing to increased sox9a expression. Force-dependent TGF-β (TGFβ) signaling is required to maintain this balance of scxa and sox9a expression. Thus, force from muscle contraction helps establish a balance of transcription factor expression that controls specialized ECM organization at the tendon enthesis and its ability to transmit force.”
” Specialized areas at the tendon-bone interface called entheses accommodate these physical constraints using a unique fibrocartilaginous extracellular matrix (ECM) that varies in composition along the length of the enthesis “
“Scx and Sox9 are expressed in opposing gradients along the length of more mature entheses in mice, with much higher levels of Sox9 closer to the bony insertion”







