Heterotopic ossification is endochondral ossification that occurs outside the bone. Understanding why it occurs can help us find ways to induce endochondral ossification within the bone. The biggest issue with inducing a new growth plate in bone is the permissive local environment criteria. The bone likely has to be degraded in some way to induce a neo-growth plate as the existing bone environment likely puts a constraining factor on growth.
Identifying the Cellular Mechanisms Leading to Heterotopic Ossification.
“Heterotopic ossification (HO) is a debilitating condition defined by the de novo development of bone within non-osseous soft tissues, and can be either hereditary or acquired. The hereditary condition, fibrodysplasia ossificans progressiva is rare but life threatening. Acquired HO is more common and results from a severe trauma that produces an environment conducive for the formation of ectopic endochondral bone. Despite continued efforts to identify the cellular and molecular events that lead to HO, the mechanisms of pathogenesis remain elusive. It has been proposed that the formation of ectopic bone requires an osteochondrogenic cell type, the presence of inductive agent(s) and a permissive local environment. To date several lineage-tracing studies have identified potential contributory populations. However, difficulties identifying cells in vivo based on the limitations of phenotypic markers, along with the absence of established in vitro HO models have made the results difficult to interpret. The purpose of this review is to critically evaluate current literature within the field in an attempt identify the cellular mechanisms required for ectopic bone formation. The major aim is to collate all current data on cell populations that have been shown to possess an osteochondrogenic potential and identify environmental conditions that may contribute to a permissive local environment. This review outlines the pathology of endochondral ossification, which is important for the development of potential HO therapies and to further our understanding of the mechanisms governing bone formation.”
“of the 80 % of war victims who suffer major extremity trauma during combat injury, approximately 64 % of these patients go on to develop some degree of HO”
“Current evidence suggests that the formation of ectopic bone in vivo requires three primary conditions: (1) a cell type capable of osteogenic differentiation, (2) the presence of inductive agents and (3) a permissive local environment”
“o date many contributory biological factors have been implicated in the aetiology, including the bone morphogenetic proteins (BMPs), inflammation, prostaglandin E2, hypercalcemia, hypoxia, abnormal nerve activity, immobilisation and dysregulation of hormones”
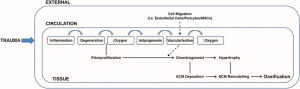
“Tissue damage leads to the infiltration of immunological cells (monocytes, neutrophils and leukocytes) through the local vasculature. Resulting fibro-proliferation of an as yet unknown cell population is accompanied by hypoxia and the generation of brown adipose tissue at the site of damage. The presence of adipose tissue is hypothesised to lower the local oxygen tension leading to the establishment of a chondrogenic environment. Neovascularisation accompanies chondrogenesis and provides an avenue through which systemic cell types (endothelial cells, pericytes etc.,) may enter the injury site, and potentially contributed to osteochondrogenic differentiation. A subsequent increase in local oxygen tension promotes chondrocyte maturation and hypertrophy. The collagenous matrix deposited by these cells is then remodelled and ossified to form endochondral bone”<-If we induce such factors in the bone we can create new growth plates in there too.
“MSCs have frequently been shown to form endochondral bone when cultured under appropriate conditions (e.g. under hypoxia and/or in the presence of TGF-β)”
“MSCs may also contribute to chondrocyte hypertrophy and the progression of HO via their immunomodulatory effects, primarily through the production of anti-inflammatory cytokines and nitric oxide (NO)”
Several cell types are listed that are capable of heterotopic ossification are likely present in bone.
“Bone marrow HSC side population Lin−/Sca-1+/cKit+/CD45+”
“Mesenchymal precursor cell (MPC) CD44+/CD49e+/CD73+/CD90+/CD105+”
“MSC CD73+/CD90+/CD105+”
“cells presenting the glutamate transporter GLAST were found to contribute to the formation of ectopic bone, and that these GLAST+ cells appeared to be distinct from the Tie2+ population”
” a significant upregulation in transcriptional activity in key osteogenesis-related genes (ALPL, BMP-2, BMP-3, COL2A1, COLL10A1, COL11A1, COMP, CSF2, CSF3, MMP8, MMP9, SMAD1 and VEGFA) in patients that developed HO compared to those who did not.”
“Heterotopic ossification (HO) is a metaplastic biological process in which there is newly formed bone in soft tissues, resulting in joint mobility deficit and pain. Different treatment modalities have been tried to prevent HO development, but there is no consensus on a therapeutic approach. Since electrical stimulation is a widely used resource in physiotherapy practice to stimulate joint mobility, with analgesic and anti-inflammatory effects, its usefulness for HO treatment was investigated. We aimed to identify the influence of electrical stimulation on induced HO in Wistar rats. Thirty-six male rats (350-390 g) were used, and all animals were anesthetized for blood sampling before HO induction, to quantify the serum alkaline phosphatase. HO induction was performed by bone marrow implantation in both quadriceps of the animals, which were then divided into 3 groups: control (CG), transcutaneous electrical nerve stimulation (TENS) group (TG), and functional electrical stimulation (FES) group (FG) with 12 rats each. All animals were anesthetized and electrically stimulated twice per week, for 35 days from induction day. After this period, another blood sample was collected and quadriceps muscles were bilaterally removed for histological and calcium analysis and the rats were killed. Calcium levels in muscles showed significantly lower results when comparing TG and FG (P<0.001) and between TG and CG (P<0.001). Qualitative histological analyses confirmed 100% HO in FG and CG, while in TG the HO was detected in 54.5% of the animals. The effects of the muscle contractions caused by FES increased HO, while anti-inflammatory effects of TENS reduced HO.”
“The formation of heterotopic bone may be due to muscle trauma (myositis ossificans). It is common in people who have undergone total hip arthroplasty , those with spinal cord injuries, and victims of head trauma, all of which often lead to long periods of immobilization of the affected limbs.”
“skeletal muscle serves as a physical safeguard for the other organs and is anatomically located immediately beneath the skin, so it represents the most damaged organ in the body. Although skeletal muscle is characterized by the presence of fatty and connective tissues that originated from nonmyogenic mesenchymal progenitors, those progenitors were initially identified in BM”
“Muscle contraction occurs by the deposition of calcium in muscle tissue, and this stimulates the sliding of actin and myosin myofibrils, which characterizes the contractile process”
“electrical stimulation helps the deposition of calcium, causes changes in oxygen content and pH, stimulates expression of growth factors, and recruits help in osteoblast migration and secretion of extracellular matrix (ECM), leading to bone formation.”
“Mechanotransduction refers to the process by which the body converts a mechanical stimulus into a cellular response”
“Osteoarthritis (OA) is associated with the metabolic syndrome, however the underlying mechanisms remain unclear. We investigated whether low density lipoprotein (LDL) accumulation leads to increased LDL uptake by synovial macrophages and affects synovial activation, cartilage destruction and enthesophyte/osteophyte formation during experimental OA in mice.
LDL receptor deficient (LDLr−/−) mice and wild type (WT) controls received a cholesterol-rich or control diet for 120 days. Experimental OA was induced by intra-articular injection of collagenase twelve weeks after start of the diet. OA knee joints and synovial wash-outs were analyzed for OA-related changes. Murine bone marrow derived macrophages were stimulated with oxidized LDL (oxLDL), whereupon growth factor presence and gene expression were analyzed.
A cholesterol-rich diet increased apolipoprotein B (ApoB) accumulation in synovial macrophages. Although increased LDL levels did not enhance thickening of the synovial lining, S100A8 expression within macrophages was increased in WT mice after receiving a cholesterol-rich diet, reflecting an elevated activation status. Both a cholesterol-rich diet and LDLr deficiency had no effect on cartilage damage; in contrast, ectopic bone formation was increased within joint ligaments (fold increase 6.7 and 6.1, respectively). Moreover, increased osteophyte size was found at the margins of the tibial plateau (4.4 fold increase after a cholesterol-rich diet and 5.3 fold increase in LDLr−/− mice). Synovial wash-outs of LDLr−/− mice and supernatants of macrophages stimulated with oxLDL led to increased transforming growth factor-beta (TGF-β) signaling compared to controls.
LDL accumulation within synovial lining cells leads to increased activation of synovium and osteophyte formation in experimental OA. OxLDL uptake by macrophages activates growth factors of the TGF-superfamily.”
“multiple injections of members of the TGF-super family, such as TGF-β or BMP-2, directly into the knee joint of the mouse caused abundant enthesophyte/osteophyte formation”
