 I was reading some random study in the field of orthopedics today and someone mentioned the condition SED, or Spondyloepiphyseal dysplasia congenita based Dwarfism, and that made me remember a few things that I had not looked at for a long time.
I was reading some random study in the field of orthopedics today and someone mentioned the condition SED, or Spondyloepiphyseal dysplasia congenita based Dwarfism, and that made me remember a few things that I had not looked at for a long time.
I remember people telling me about quack-pseudo-scientific ideas on how to treat SED before on other websites, but I wondered whether the Patent databases had anything written about treating it, in a more scientifically legitimate way. So I googled “Spondyloepiphyseal Dysplasia Congenita” into the Google Patent database to see what would come up.
Some of the first few results were obviously crazy, like the following “Drug composition for treating spondyloepiphyseal dysplasia – CN 103142900 A”. This patent apparently was only filed in the China based database, and it is based on a combination of strange vegetables, minerals, and plants. The idea from this ancient formulation I would guess is to reduce the curvature of the spinal or straighten it out, which would obviously give the person a little bit of height increase. However, I would not be willing to put money on an idea based on Traditional Chinese Medicine practices.
It would be the next search result, which really caught my eye. Refer to the patent “Variants of C-Type Natriuretic Peptide – US 20100297021 A1″. This was a patent which I don’t believe I have talked about before, and the name of it would not be an indication to most people searching the Patent Database that it is anything of importance, but this patent is basically a patent for a way to help people become taller.
Tyler and I have in our research throughout the years have seen multiple similar patents working on the similar idea which was also for increasing stature.
Refer to the Abstract below….
The present disclosure provides variants of C-type natriuretic peptide (CNP), pharmaceutical compositions comprising CNP variants, and methods of making CNP variants. The CNP variants are useful as therapeutic agents for the treatment of diseases responsive to CNP, including but not limited to bone-related disorders, such as skeletal dysplasias (e.g., achondroplasia), and vascular smooth muscle disorders (e.g., restenosis and arteriosclerosis).
The Inventor is a Daniel Wendt from Biomarin Pharmaceutical. If we need to remember, Biomarin was the company which made the BMN 111 compound, which is used to treat childhood achondroplasia. I remember reading about this drug maybe a year ago on a website that focused specifically on how to help cope with a child who has the condition.
As one can see, this patent was filed back in 2010 and it has been filed in the USA, Canada, Europe, China, and the World Patent Database System. If I was to guess, I probably had linked to this patent multiple times before, but never really read the patent thoroughly..
Refer to the sections in the patent which I will highlight below…
“….In contrast, mice engineered to produce elevated levels of CNP display elongated long bones and vertebrae.”
I would learn further that in the growth plate, the proliferative zone expresses a compound called NPR-B while the hypertrophy zone expresses NPR-C. CNP (which stands for C-Type Natriuretic Peptide) is an agonist for the NPR-B. Agonist just means some type of chemical or protein that assists or increases a biochemical process. Further down the chain of chemical processes (which in the chemical/medical industry call downstream), the CNP and NPR-B pathway causes the FGFR3 pathway to be blocked. Remember that Achondroplasia is most often caused by a mutation of the FGFR3 causing it to be overstimulated or extra-sensitive. In the FGFR3 pathway, there is a step in the MAPK section. It seems that the CNP/NPR-B disrupts the pathway at MAPK, causing the FGFR3 to become inhibited, thus removing the stunted height morphology.
Refer to the section below from the patent….
“In humans activating mutations of FGFR-3 are the primary cause of genetic dwarfism. Mice having activated FGFR-3 serve as a model of achondroplasia, the most common form of the skeletal dysplasias, and overexpression of CNP rescues these animals from dwarfism. Accordingly, CNP and functional variants of CNP are potential therapeutics for treatment of the various skeletal dysplasias.”
Something that I would further learn is that the plasma-half life of CNP in the human blood stream is very short, only 2.6 minutes (in-vivo). This means to get this biomedical technique to work out, the CNP would need to be continuously pumped into the subject’s body. Apparently the basic level of CNP in the human body is around 5 picoMolar (5*10^(-9) Molar). The level of CNP must be higher than this concentration.
Refer also to more sections from the patent…
“…In a further embodiment, the CNP variants are useful for increasing the size of the growth plate of a bone (e.g., a limb bone). In another embodiment, the CNP variants are useful for elongating a bone or increasing long bone growth. In still another embodiment, the CNP variants are useful for enhancing matrix production, proliferation and differentiation of chondrocytes.”
Apparently to make the femur longer at the highest rate, you want to use a variant of CNP known as N-terminal PEGylated CNP variant.
“…Ex vivo studies of cultures of mouse bones indicated that CNP37 was delivered to the growth plate and was able to increase chondrocyte cellularity and hypertrophy, which are associated with growth plate expansion and longitudinal bone growth.”
“…These results demonstrate that CNP variants of the disclosure penetrate into the growth plate of wild-type and achondroplastic animals, increase the number and size of chondrocytes, increase the thickness of the proliferating zone and the hypertrophic zone of the growth plate, and increase longitudinal bone growth in treated wild-type and achondroplasic animals. Therefore, the CNP variants are useful for stimulating bone growth in achondroplastic subjects.”
There apparently was even news stories published in the Major Online News Websites talking about this drug/invention. (Note: Yes, I am fully aware that I am assuming that the patent I found is referring to the Vosoritide/BMN 111 chemical compound)
Drug Accelerated Growth in Children With Dwarfism, Pharmaceutical Firm Says – 6/17/2015 – The news reported that children would be growing around 6 cm/year than 4/cm by taking the drug. The other name for BMN 111 is vosoritide. There has been questions about the efficacy of this chemical after the first year of use, and whether after the first year the drug would still be as effective as the first year. In addition, it seems that people who did not suffer from Achondroplasia did not get any type of increased bone longitudinal growth benefit from using it. Since the article was written back in June of just this year, the drug is now in Phase 3 Trials, where around 50-150 children will be subjects for this new treatment.
BioMarin drug boosts growth in children with dwarfism – the source here seems to make a correction on something the previous source said, which is that the 6 cm of increased height is actually from just 6 months of tabulated data, not a full year.
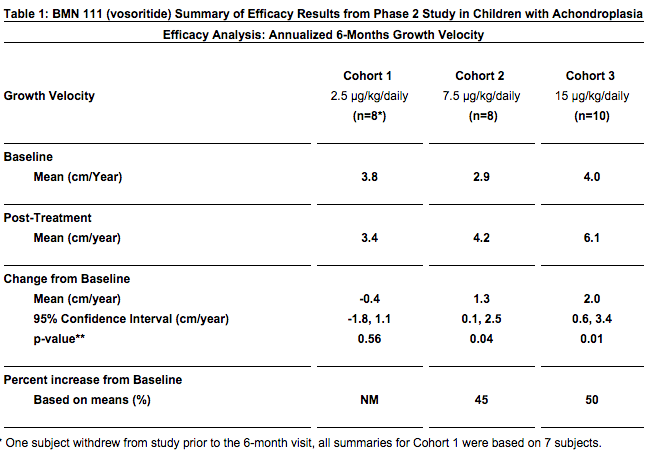
BMN 111 (vosoritide) Improves Growth Velocity in Children With Achondroplasia in Phase 2 Study – This source suggest that the efficacy of BMN 111 will continue past the 6 month range in Phase 2 –
- There was a dose-related increase in urinary excretion of cGMP measured over the 6 month duration of the study. cGMP is a biochemical marker that may indicate that BMN 111’s biological effect will continue beyond 6 months.
Refer to the chart from the source below….
It would seem that National Health Industries and Organizations will not fund for a condition unless it has been diagnosed as a real medical condition or a disability. Short stature is not technically a disability, but having achondroplasia or dwarfism does qualify as a medical condition. This is where biomedical research will go and the funding will flow towards.
If you guys are interested in learning more about this compound, just type in the common name for it, Vosoritide or BMN 111. Biomarin even has itself listed on the Nasdaq under the acronym BMRN.

Would it work with closed plates?
Are that good news for us because I don’t see the ”Big Breaktrough” that you usually write when something is good for us. Does that mean it could theoretically help People with closed growth plates to get taller? and how much taller? also what will be the costs of this drug?
More answers pls,can you do that somewhere
Also is this method better than LL or Stem cells techic?
Treatment Gives Dwarf Mice a Growth Spurt:
http://news.sciencemag.org/biology/2013/09/treatment-gives-dwarf-mice-growth-spurt