Understanding Growth Plate Fusion(and growth plate senescence) will help us understand if these processes can be reversed. And just as a note I am still working on devices and methodology to grow taller.
A Computed Microtomography Method for Understanding Epiphyseal Growth Plate Fusion.
“The epiphyseal growth plate is a developmental region responsible for linear bone growth, in which chondrocytes undertake a tightly regulated series of biological processes. Concomitant with the cessation of growth and sexual maturation, the human growth plate undergoes progressive narrowing, and ultimately disappears. Despite the crucial role of this growth plate fusion “bridging” event, the precise mechanisms by which it is governed are complex and yet to be established. Progress is hindered by the current methods for growth plate visualization; these are invasive and largely rely on histological procedures. Here, we describe our non-invasive method utilizing synchrotron X-ray computed microtomography for the examination of growth plate bridging, which ultimately leads to its closure coincident with termination of further longitudinal bone growth. We then apply this method to a dataset obtained from a benchtop micro computed tomography scanner to highlight its potential for wide usage. Furthermore, we conduct finite element modeling at the micron-scale to reveal the effects of growth plate bridging on local tissue mechanics. Employment of these 3D analyses of growth plate bone bridging is likely to advance our understanding of the physiological mechanisms that control growth plate fusion.”
“It is the terminally differentiated hypertrophic chondrocyte, which mineralizes its surrounding extracellular matrix. This process, thought to involve membrane-limited matrix vesicles, is biphasic and tightly regulated by a number of enzymes and factors including alkaline phosphatase (Alpl), PHOSPHO1, the ankylosis protein (Ank), ecto-nucleotide pyrophosphatase/phosphodiesterase-1 (Enpp1) ”
“as growth slows, the human growth plate undergoes progressive narrowing as bony bridges form and span its width. This ultimately leads to complete growth plate closure and the cessation of human growth. These bone bridges are also known to form upon growth plate injury, thought to be through an intramembranous ossification mechanism”
“in two genetic mutations resulting in estrogen deficiency (in the estrogen-receptor gene, and in the CYP19 gene), the growth plate fails to fuse and growth persists, albeit rather slowly, into adulthood”
“Evidence from studies in both humans and rats revealed the cessation of growth long before any histological evidence of growth plate fusion, suggesting that epiphyseal fusion is a marker of growth cessation and not its cause “<-Thus it may be possible to renew growth plate growth by reversing cessation and not fusion.
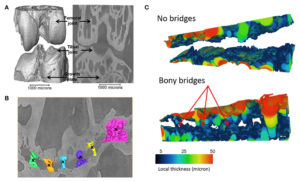
“young (8 weeks old) CBA wild-type mice, growth plate bridging is associated with locations that contain high local von Mises stresses. Moreover, we reveal that with aging an increased number and density of growth plate bridges is observed”
” in wild-type mice, increased growth plate bridging translates into increased stresses in the bone directly beneath the growth plate.”<-So maybe these stresses contribute to growth plate closure.
“At 8 weeks, few bridges are detected and overall the growth plate is squeezed in a “sandwich” configuration. This suggests that compressive hydrostatic stresses are engendered across major volumes and that higher shear stresses are generated only at the peripheral edges of the growth plate. Yet, the results of numerous mechanobiological models support that growth and ossification is accelerated by tensile strain (or shear stresses) and that cartilage tends to be maintained by hydrostatic compressive stress“<-so for a longer growth period we need to encourage hydrostatic compressive stress.