Novel Treatment Options in Childhood Bone Diseases
“Several novel treatment options have recently become available in childhood bone diseases. The purpose of this article is to provide an update on some of the therapeutic agents used in the treatment of pediatric osteoporosis, X-linked hypophosphatemic rickets, and achondroplasia (ACH){this is what we’re interested in as it’s related to height}. Summary: Vitamin D3 and Ca supplementation remains the basis of childhood osteoporosis treatment. Bisphosphonate (BP) therapy is the main antiresorptive therapeutic option, while denosumab, a human monoclonal IgG2 antibody with high affinity and specificity for a primary regulator of bone resorption – RANKL, represents a possible alternative. Its potent inhibition of bone resorption and turnover process leads to continuous increase of bone mineral density throughout the treatment also in the pediatric population. With a half-life much shorter than BPs, its effects are rapidly reversible upon discontinuation. Safety and dosing concerns in children remain. Novel treatment options have recently become available in two rare bone diseases. Burosumab, a monoclonal antibody against FGF-23{FGF23 has an impact on height but there’s mixed evidence on whether it’s good or bad therefore Burosumab may have an impact on height too}, has been approved for the treatment of children with X-linked hypophosphatemic rickets older than 1 year. It presents an effective, more etiology-based treatment for rickets compared to conventional therapy, without the need for multiple daily oral phosphate supplementation. Its long-term efficacy and safety are currently being investigated. After years of anticipation, a novel treatment option for ACH has become available. C-type natriuretic peptide analog vosoritide effectively increases proportional growth and has a reasonable safety profile in children >2 years. Its effect on other features of the disease and the final height is yet to be determined.{studies show that vosoritide definitely increases growth rate but there’s yet a study that shows it’s impact on adult height; Micheal’s thoughts on vosoritide; I also speculate that CNP could help with longitudinal bone growth in adults if one as an adjunct to other methods or maybe to help grow via the cartilage in the spine or knee etc; CNP increases the proliferation of chondrocytes in general} Several other treatment options for ACH exploring different therapeutic approaches are currently being investigated. Key Messages: Denosumab is effective in the treatment of childhood-onset osteoporosis; however, further studies are necessary to determine the optimal treatment protocol. Burosumab is more etiology-based and convenient in comparison to conventional treatment of X-linked hypophospha-temic rickets in children and adults. Vosoritide importantly changes the natural course of achondroplasia, at least in the short term.”
“Burosumab is the first etiologic treatment option that actively increases phosphate levels while also decreasing FGF-23 actions in XLH. It is a monoclonal IgG1 antibody that suppresses the actions of FGF-23. FGF-23 is the key phosphaturic hormone and acts as a regulator of phosphate homeostasis. It mediates its actions by binding to its cofactor alpha-Klotho and FGF-receptor 1 (FGFR1), through which it inhibits phosphate reabsorption in the kidney via downregulation of the sodium-dependent phosphate transporters (NaPi-2a and NaPi-2c) in proximal renal tubules. Additionally, it suppresses renal 1α-hydroxylase (CYP27B1) and activates 24-hydroxylase (CYP24A1), both of which contribute to lowering serum concentrations of 1,25-dihydroxy cholecalciferol and thus reduce intestinal uptake of phosphate. The elimination of burosumab follows the endogenous immunoglobulin degradation pathway”
“In another phase 3 study (study identifier NCT02915705) burosumab treatment was superior to conventional therapy regarding growth velocity and disease progression determined by RSS”<-burosumab’s impact on growth velocity suggests that it may impact height. IF you look at the study menionted figure 4,Height was increased by about 0.2cm.
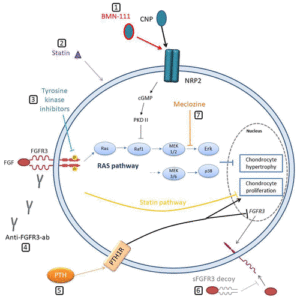
“Excessive FGFR3 activation results in downstream activation of multiple intracellular signaling pathways, leading to intensified inhibition of cartilage tissue formation at the level of chondrocyte proliferation (via STAT1), hypertrophy, differentiation, and synthesis of the extracellular matrix (via Erk-MAPK signaling pathway) “
“Growth hormone supplementation has not shown promising results and is not viewed as a standard treatment for ACH. The progress in the understanding of ACH pathogenesis has led to the development of many potential therapeutic strategies for modulating excessive FGFR3 activation. Approaches are varied and include inhibiting the tyrosine kinase activity of FGFR3 (infigratinib), producing artificial FGFR3 as a decoy for FGF ligand (recifercept), inhibition of FGFR3 downstream signaling pathways (meclizine, C-type natriuretic peptide [CNP] analogs), modulation of growth via natriuretic peptide receptor 2 (NPR2) receptor (CNP analogs) and use of aptamers or monoclonal antibodies to prevent binding of FGF to its receptor (aptamer RBM-007, vofatamab). The investigations into analogs of CNP, especially vosoritide, are currently the most advanced”
“Vosoritide is a recombinant CNP analog. Endogenous CNP and its action on the growth plate through NPR-B are recognized as one of the important regulating mechanisms of longitudinal bone growth. Coupled with NPR-B, CNP antagonizes downstream FGFR3 signaling by inhibiting the Erk-MAPK signaling pathway at the level of Raf. This leads to chondrocyte proliferation, differentiation and increases the extracellular matrix synthesis. CNP-targeted overexpression in the cartilage or its continuous delivery by intravenous infusion has shown normalization of the impaired bone growth in mouse models with ACH”
” In August 2021, results of the extension phase 3 clinical trial in children with ACH aged between 5 and 18, receiving vosoritide 15 μg/kg once daily in subcutaneous injection, were published. An increase in annualized growth velocity was observed, with 3.52 cm of height gain over a 2-year treatment period in comparison to untreated patients. In addition, improvement in the proportionality of body segments and no acceleration of the bone maturation process (determined by bone age assessment) was observed{this is a positive indicator that the treatment will increase adult height}“
“Recent preclinical data in healthy cynomolgus monkeys showed that treatment with TransCon CNP subcutaneously once per week resulted in significant growth increases in body, tail, and long bones compared to controls. An increase in height was also more pronounced in comparison to the animals receiving a daily dose of CNP analog with the same amino acid sequence as vosoritide (5% vs. 3%, respectively), and no significant changes in bone quality were observed with both treatments. Moreover, sustained CNP release resulted in lower systemic CNP peak levels and has not been associated with adverse cardiovascular effects in monkeys treated with repeated weekly doses up to 100 μg/kg”
According to A long-acting C-natriuretic peptide for achondroplasia, “CNP-38 was slowly released into the systemic circulation and showed biphasic elimination pharmacokinetics with terminal half-lives of ∼200 and ∼600 h. Both preparations increased growth of mice comparable to or exceeding that produced by daily vosoritide.”
So both vosoritide and Transcon CNP increase height during development and I suspect may have some applications for adults as well. Burosomab and the other FGFR3 inhibitors likely have impact on height as well.
{Note I accidentally made this post in Michael’s account}