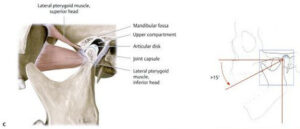
This is a huge find because it means that we may actually be able to pull on bones to make them longer. Now it’s easier to deform bones by bending than it is by pulling on them but bending generates both tensile and compressive forces so you’d have to find a way to bend the bone in two parts at once to balance the compressive forces to achieve length. Lateral compressive forces are axial tensile forces so this is why something like tapping can be beneficial to achieve length. Don’t think that sleeping in a “rack” position can achieve this kind of pull the problem is that the pull forces are observed muscle by the muscles and ligaments which only pull the bone at the enthesis(which not surprising is longer and thicker than other parts of the bone), you’d have to find a way to generate pull directly on the bone itself. Also, consider that sleeping in the rack position could potentially cut off circulation when you’re not awake to do anything about it.
If corsets generated bone shape increase via plastic deformation rather than traditional bone modeling with osteoclast absorption and osteoblast deposition that is a huge find because bone modeling is not likely to result in an increase in length unless osteoblast deposition occurs at the longitudinal ends of bones.
Here’s the citation:
Corsets and Skeletal Deformities: Anthropological Study
“The skeletons of 19th century corseted women were studied to see how their ribcages were flexibly bent into a more tapered shape from the corset. From the photos, you can see literal ‘bends’ in the ribs where the pressure from the corset formed the ribs into the shape of a circle. Also, the spinous processes seemed to be affected too: spinous processes are the small “spikes” humans have on their vertebrae; they look like spikes down a lizard’s back, but in humans these are small and one can occasionally see or feel them as the ‘bumps’ along one’s back. In the skeletons that showed rib shaping from a corset, these same skeletons also had “spikes” in the upper back that bent downward and overlapped like snaggleteeth.”
Corsets are basically lateral compressive loading on the ribs so should generate a minor longitudinal tensile force as well. it’s not surprising that corsets also generate torsional and rotational forces which explain why the spinous process of the vertebrae were deformed. But ribs are bent so it would be easier to generate stronger mechanical forces than it would be in straighter long bones.
“Gibson first chose skeletons showing classic deformation of the ribcage from the pressure of the corset (which in O’Followell’s study, pressure from the corset is shown to be measured up to 80psi). The ribs were more circular compared to an anatomically “normal” human ribcage (the “control” ribcage).” 1 psi is about 0.007 MPa. Or about 0.56 MPa total.
Rickets can also cause deformation of the rib bones “rickets flattens the curves of the ribcage, and most of the ‘bend’ occurs at the costal joints, especially at the sternum (“pigeon chest” is common) – and in extreme cases of rickets, the pressure from one’s own arms laying at the side of the body can cause the ribcage to cave in at each side.”
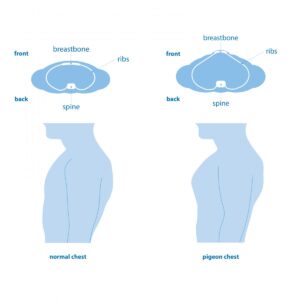 A pigeon chest looks longer than a regular chest so if we look at the principles that make a pigeon chest that may translate into longer bones. Rickets results in less height but more bended bones.
A pigeon chest looks longer than a regular chest so if we look at the principles that make a pigeon chest that may translate into longer bones. Rickets results in less height but more bended bones.
 Since rickets bones would more malleable it would be interesting to try a bone loading deformation routine on someone with rickets.
Since rickets bones would more malleable it would be interesting to try a bone loading deformation routine on someone with rickets.
“In the French [corset] skeletons, instead of seeing “flattened curves”, Gibson noted that the ribcage was more rounded, such that when the dimensions were measured, the coronal (front-to-back) and sagittal (side-to-side) diameters were identical (or close to it). Also, the area of the rib with the greatest bending was closer to the back of the body, not at the sides where the arms would be. Gibson said that formation was seen as high as the 4th rib (imagine right up in the armpit) and the corset molded each successive rib consistently and uniformly. The floating ribs (11th and 12th) were sometimes affected even more.”
“I found Figure 6 of the article to be of particular interest, where a single pair of ribs shows plastic deformation of the rib (actual bending near the back), a broken area that had healed later in life, and a post-mortem breakage (obviously not healed), showing how different all of them appear.” Now the usage of the term plastic deformation in the study studied may need indicate that plastic deformation actually occurred it may have been traditional bone modeling rather than mechanical loading resulting in permanent change in shape.
” throughout the thoracic spine, normal spinous processes are already angled slightly downward, although may not necessarily overlap. It would not be impossible for these processes to deform with regular pressure and a predisposition to softer bones,”<-it would be interesting if different shaped and designed corsets would have different affects on the shape of the spinous process.
Something like scoliosis bracing has always thought to only be beneficial during development as as a means to alter spinal development mainly through altering growth via compression and tension on the growth plate. Something like artificial cranial deformation was thought only to work in the youth. If something like the corset can change the spine as an adult than maybe bracing can too.
The key step is catabolism. HGH increases bone turnover which involves both catabolism and anabolism of bone. So we need to not only stimulate bone growth but bone degradation as well to allow the bone growth to be of a less mature cell type.