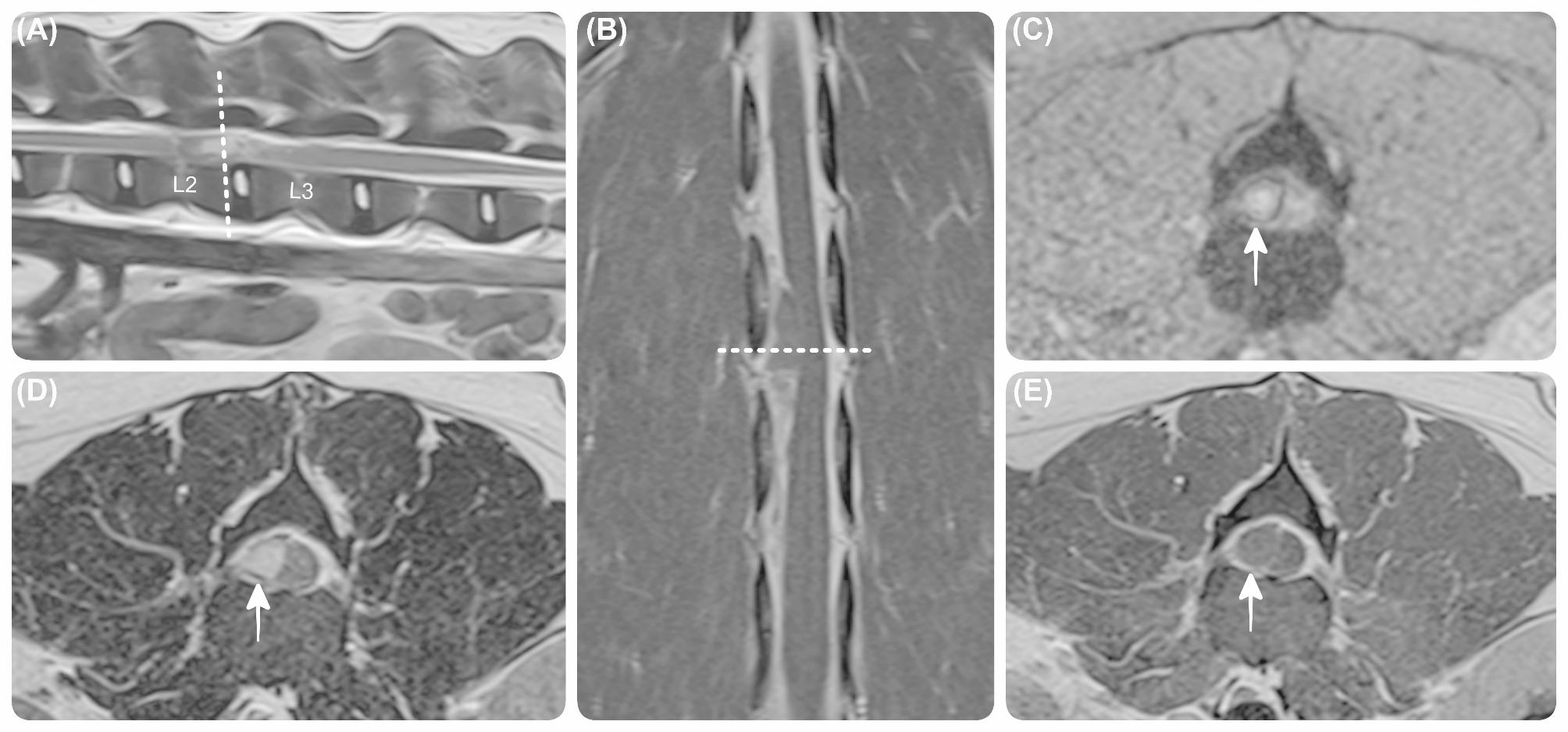
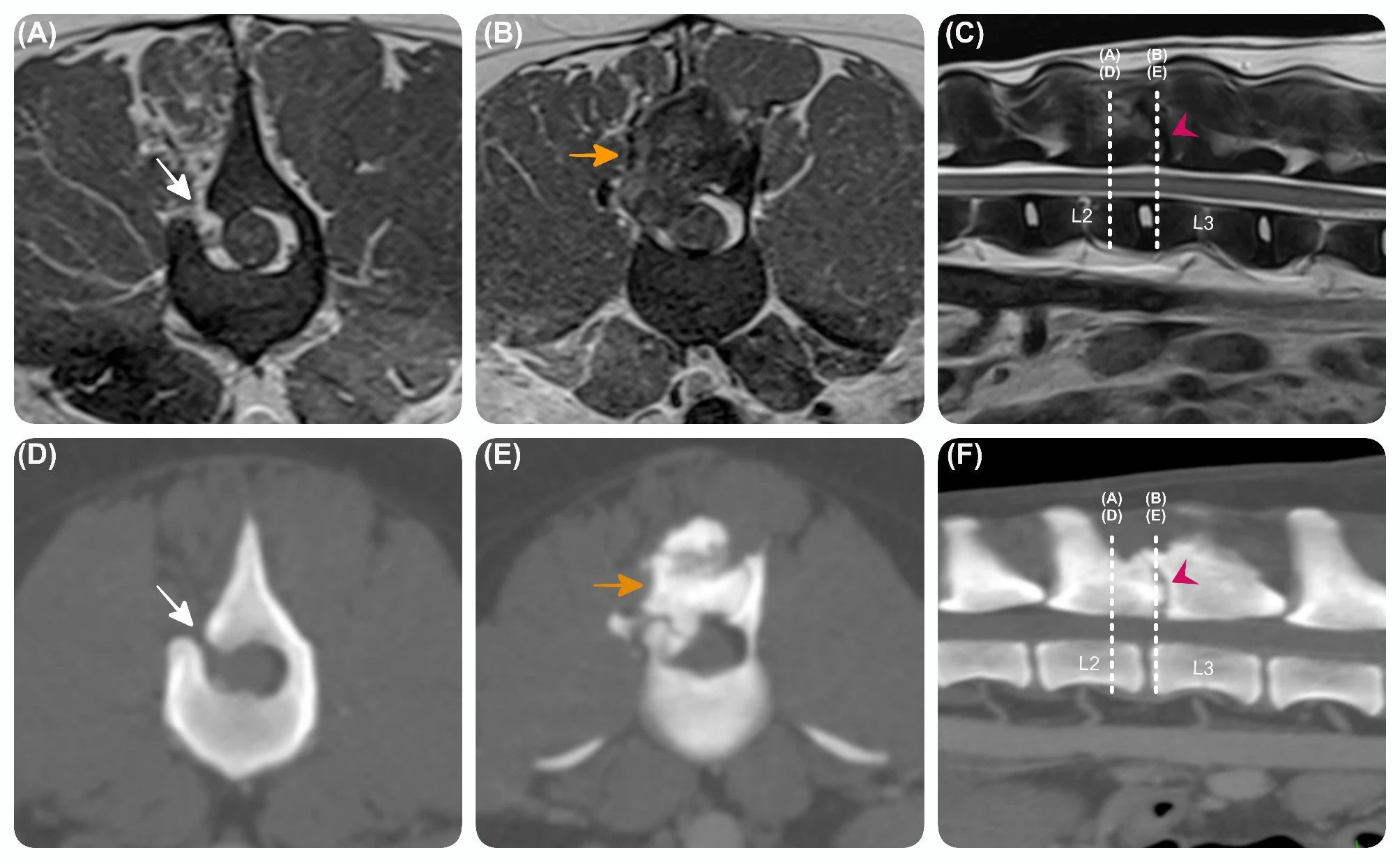
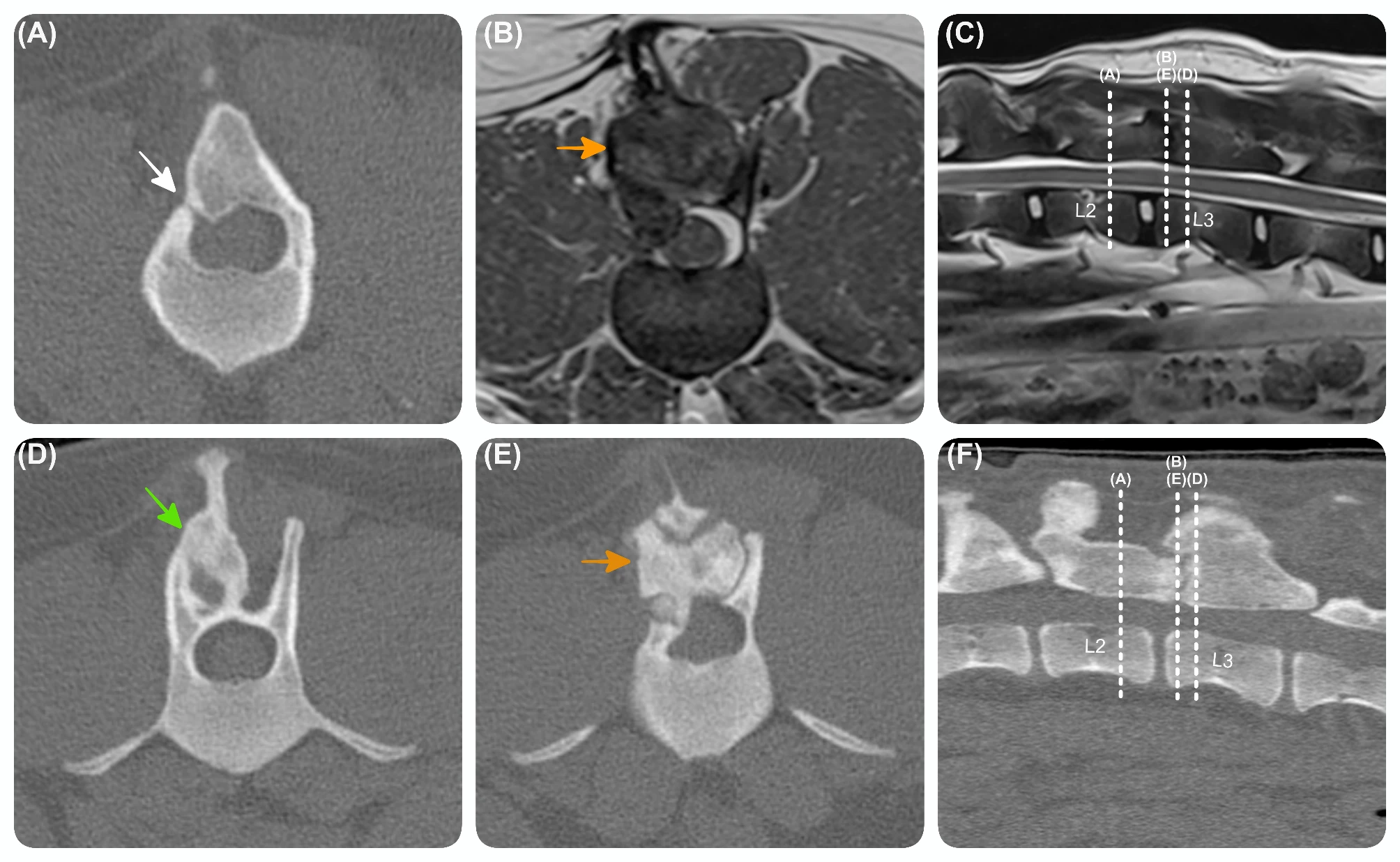
Here’s the new LSJL method I’m trying,
Here’s the thread where I share my progress and my thoughts.
The method isn’t quite lateral synovial joint loading but it is based on the same science. I remember watching a Hiroki Yokota where he explains that he got the idea for lateral loading the synovial joints because of a water bottle. Axial loading does not drive a lot of fluid through the bottle but lateral loading does. If you tap on the axial end of the water bottle not a lot of water travels through the battle. If you tap on the lateral end water travels far more.
This is the lecture where he made that point but I can’t seem to access it.
The problem with lateral loading of the epiphysis is that there seemed to be a limit to be bone deformation of the epiphysis. Also, the epiphyseal line may reduce the ability for the fluid forces to be transmitted through the rest of the bone. Also, there was problems with slippage of the clamp.
Most fluid forces generated in the bone are generated via muscular contractions and axial loading/impact. There is a threshold that has to be generated in terms of fluid forces(Hydrostatic pressure, fluid flow, etc.) that needs to be surpassed to potentially induce longitudinal bone growth i.e. degradation of cortical bone, a chondrogenic microenviroenvironment, mesenchymal condensation, etc.
Lateral impact doesn’t really occur physiologically, I looked at muay thai shin condition and it seemed to be mostly surface level not really affecting deep within the bone. Tennis may cause a little bit of lateral impact within the bone. But it’s dependent on the force of the ball in the racket transmitting into the arm. It’s not easily reproducible. So lateral impact on the bone is not really something that occurs physiologically.
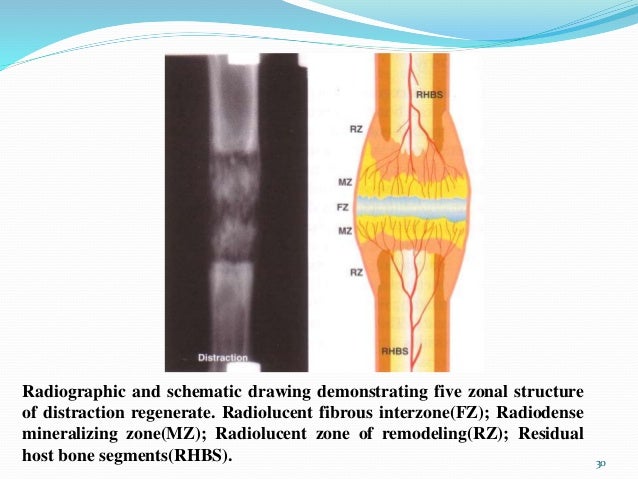
In distraction osteogenesis, one of the key steps is a hematomma allowing for bone regeneration to occur. This establishes that the bone marrow is a very powerful stimulating force on the bone.
The goal is to try to mimic this mechanical stimuli without actually breaking the bone and the method is to try to generate as much fluid pressure on the bone as possible.
We could combine lateral impact, with epiphyseal clamping, and muscular contraction to try to increase the fluid forces of the bone to be higher. We want the tapping to be on the cortical bone of diaphysis so it stimulates the marrow cavity. That the marrow cavity turns to fat yellow bone marrow should be a big clue. If something changes from development it it something we likely want to try to revert to get to an early developmental stage.
Now how to prove that this occurs?
Method 1: Self testing.
Method 2: Look inside the bone to see how it responds to fluid forces(it’s been done with hydrostatic pressure and interstitial fluid flow but mostly at a cellular level). We’d ideally want to see degradation of cortical bone(can measure via certain osteoclast markers), chondrogenic differentiation, and mesenchymal condensation. This would take possibly years in the lab.
But there have been numerous studies showing the power of fluid forces in bone. Lateral impact generates the most fluid forces in bone. Fluid forces that have been shown to induce chondrogenic differentiation and powerful regeneration.
To contradict this theory, provide evidence that:
- Lateral impact on the bone does not generate the most fluid forces in the bone
- lateral impact already occurs physiologically in an activity or sport
- No amount of lateral impact could pass the pressure/other thresholds to stimulate longitudinal bone growth.