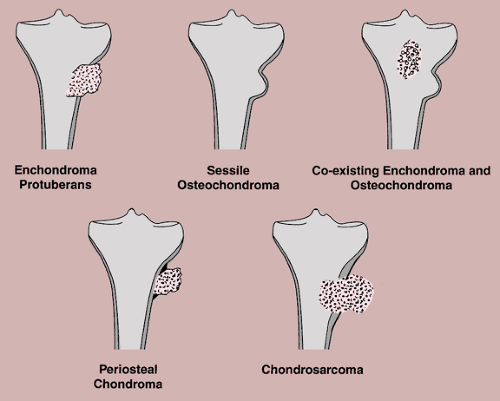
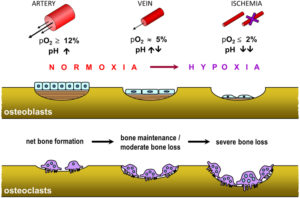
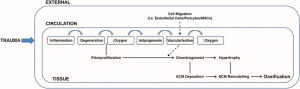
The first Yokota/Zhang patent doesn’t provide that much insight but the second study provides a potential device that gives an alternative method of bone lengthening. The study is confusing so I’d appreciate any second opinions.
Mechanical bone loading to reduce arthritic pain
“mechanical loading of the knee to downregulate nerve growth factor beta (NGFb), which is believed to be a major cause of pain in arthritic joints.”
“the joint loading may be performed at between 0.5 N and IO N, preferably at 1 N, and the fluid flow may be performed at, for example, 5 dyn/cm2. In one aspect, the results described herein suggest that gentle knee loading analogous to massage therapy is beneficial not only to enhancing bone formation and accelerating wound healing but also to preventing NGFP-induced nerve growth and pain perception in cartilage.”
” it has been recently suggested that a consequence of compressive loading is production of hydrostatic pressure as well as fluid flow to cartilage.”<-Hydrostatic pressure in bone could be a key to induce neo-growth plates.
“In osteoarthritis, chondrocytes are known to be exposed to flow shear presumably due primarily to synovial fluid and high amplitude of fluid flow reproduces the hallmarks of osteoarthritis in vitro. The frequency of 5 Hz might not be representative of massage to humans by hands but more pertinent to those by vibrator for foot massage. In another embodiment as described herein, the levels of loading in vivo have been optimized herein to produce anabolic response in the bone and cartilage.”
“Cyclic compression was applied to the mouse right knee using a custom-made piezoelectric loading device following reported methods. The mouse was mask-anesthetized using 2% isoflurane, and lateral loads to the knee were applied for 5 min at 5 Hz with a peak-to-peak force of 1 and 3 N.”
“Knee loading at 1 N but not at 3 N decreased the phosphorylation level of p38 (p- p38) in the cartilage”
“it was discovered herein that joint loading, illustratively, of a knee at 1 N, reduced mRNA levels of NGF and its low affinity receptor, p75 in cartilage and subchondral bone. Additionally, it was discovered that, in cartilage, joint loading, illustratively, of a knee at 1 N, reduced the phosphorylation level of p38 MAPK (p38-p) and activity of Racl GTPase. Additionally, it was discovered that, fluid flow at, for example, 5 and 10 dyn/cm2, reduced mRNA levels of NGFP and p75{neuron related gene} in C28/I2 human chondrocytes.”
“Nerves are known to exist in trabecular bone of the epiphysis, and are believed to grow in response to NGF{it’s possible that the growth of these nerves could affect height growth} . Although healthy cartilage is not believed to consist of vascular or neural tissues, arthritic cartilage is believed to lose its ability to remain aneural and avascular. It has been reported that dynamic loading to cartilage evokes stimulation of matrix synthesis{Could enough matrix synthesis increase height}, as well as regulation of enzymatic activities of matrix metalloproteinases. In addition to the reported regulatory role in matrix homeostasis, in one embodiment of the invention herein the results herein point out that mechanical stimuli at moderate amplitudes regulate transcription of NGF and its receptor in cartilage and chondrocytes.”
“both gentle mechanical loading and salubrinal share the Racl -mediated signaling pathway for – mRNA expression of NGF. In myocardial remodeling, it is reported that deficiency of Racl reduces stress to the endoplasmic reticulum. Since the elevated phosphorylation level of eIF2ot by salubrinal also suppresses stress to the endoplasmic reticulum, the observed linkage of salubrinal to Racl appears to be consistent with downregulation of NGF .” Note that an increase in Rac1 expression was linked to an increase in chondrogenic marker genes. This suggests that gentle mechanical loading may not be best for inducing exogenic bone mesenchymal chondrogenesis(neo-growth plate) and more extreme load may be needed.
This next paper is listed at the end as a related method:
System and Method for Joint Restoration by Extracapsular Means
“A system and method for joint restoration by extracapsular means includes an actuator operable to apply a force to a portion of a bone to effect a change in the joint space geometry. One embodiment of the system includes an actuator operable to apply a cyclic loading to subchondral bone of a femur, wherein loads of a predetermined magnitude are alternately applied and released. Between periods of cyclic loading, rest periods are provided where no load is applied. Over time, the femoral joint surface is remodeled in accordance with the location, direction, magnitude, and frequency of the loading.”
“Osteocytes sense the increased strain environment, and respond accordingly. When bone tissue is damaged as in the micro-cracking that occurs in the presence of excessive stress or strain, osteoclasts remove the necrotic osteocytes. This activates growth factors held in the osteocytes, such as bone morphogenic protein (BMP) or transforming growth factor (TGF) beta 1.”
“At sufficiently high stress levels, deformation will occur with time, leading to “creep-failure”, or deformation that does not recover once the load is removed. The creep response of bone is significantly larger in younger bones as compared to older bones.”
“Similarly, when bone is measured on a large scale, it exhibits very classical (single elastic constant) behavior, but when the scale is reduced down to the trabecular level or below, the behavior becomes much more viscoelastic in nature, and tends to follow a Cosserat (multiple elastic constants) curve. This allows for much higher than predicted (by the classical approach) strain limits before failure occurs. In order for bone formation to be initiated, the magnitude of mechanical strain of the bone must surpass some threshold. Therefore, for restorative remodeling to occur, this threshold must be exceeded, while not causing failure”
” Trabecular bone can be found inside the condylar region of a femur, and alongside the cortical bone. The trabecular bone transfers the loads from the subchondral bone to the cortical bone, and the subchondral bone is that bone which supports the articular regions of the joint surfaces. Each different type of bone may undergo different deformation mechanisms. For example, cortical bone in particular exhibits “cement line slippage” between the osteons, which accounts for an ISF type (almost viscoelastic) behavior when applied to localized regions. This is typically considered the reason bone is a “tough, non-brittle” material. It is also a response that is dependent on the direction of the applied load-a result of the oriented structure of bone”
” a more rapid load onset results in a more rapid bone change. Conversely, a slower application of a load results in a smaller change, but thickening of the bone to handle the higher stress. Thus, a static load may build more dense bone, but a dynamic load may cause greater overall deformation of the bone.”<-Thus we should probably try to make sure that the clamping is the least static possible. Constantly increasing clamping force is one way.
” the system components described herein can take advantage of the properties of bone that allow the bone to deform under constant stress via a “creep” or plastic deformation mechanism. The system components can push on the underside—e.g., the trabecular side—of the deformed subchondral bone, forcing a change of surface dimension on the joint surface (opposing) side of the subchondral bone. The subchondral bone may be softened to facilitate the reshaping process by drilling, cracking, laser etching, ultrasonically, biologically or by chemically treating the subchondral or the underlying cancellous bone, or by any other means in conjunction with the use of the system of the present invention, either to facilitate the initial movement, or during subsequent treatments. The devices according to the present invention may be permanently implanted in the bone, or can be removed after the desired results are obtained.”<-Can we induce a similar plastic deformation mechanism but in order to increase height.
“the term “static load” as used herein does not imply that a load that can or will never change; rather, the term refers to a load that is either constant for some period of time, or a load that is applied so slowly as to approximate a constant load. This is distinguished from a dynamic load, which may be a single load applied very quickly, or may be a cyclic load of constant amplitudes and/or frequency, or one of varying amplitudes and/or frequency.”<-So we may want to rapidly clamp then unclamp in order to get a single load applied rapidly.
” the present invention has applications where shortening or lengthening of bone is desired to restore a normal joint geometry, and little or no joint surface remodeling is required. For example, a system including piezoelectric actuators can be applied to one or both sides of a joint to correct an angular displacement. ”
” For example FIG. 14 shows a tibia 76 having a system 78 in accordance with the present invention attached to it. The system 78 includes a linear actuator 80, which can be used to apply a static load, a cyclic load, or some combination thereof to the tibia 76. When a system, such as the system 78, includes two or more such actuators, one can be inserted in the cortical region and over time “grow” one side—e.g., the lateral side—and another can be inserted on the medial side to contract the bone. This effects an angular change at the joint line, and restores a more appropriate mechanical joint alignment.”<-For our purposes, we’d just set the two actuators to length bone.
“FIG. 14 shows a system in accordance with another embodiment of the present invention, the system including a linear piezoelectric actuator to increase the length of a tibia;”
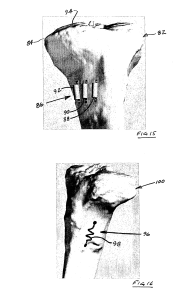
“FIG. 15 shows a system in accordance with another embodiment of the present invention, the system including a plurality of linear piezoelectric actuators to increase the length of a tibia as an alternative to an osteotomy”
Nowhere does it state that this would be limited to individuals with opten growth plates and in fact there are no visible growth plates on this bone.
This is an example of a linear actuator:
Note that I have no idea whether this actuator is sufficient in any way to provide a lengthening force on the bone. It is just an example.
Note that the linear actuator is used in addition to the invention. It seems that MIchael has considered using a linear actuator for a height increase device before.
” a swelling memory polymer can be used to provide expansion in a predetermined direction to a predetermined volume, thereby exerting pressure against the containing tissues. Shape memory alloys, such as Nitinol (Ni-TI) can also be used. Such alloys, commonly used in bone staples, can be formed as “muscle wires” and inserted into the cortical bone, where they lengthen in response to outside stimuli.”
“A shape memory alloy could also be formed as a spring, and configured to lengthen (or contract) upon application of an electrical current, for example, an 80 mA current at 20C”
This is how he describes inserting the device into the bone:
“creating an aperture[opening] in the bone proximate the articular surface, thereby making accessible an internal portion of the bone generally opposite the articular surface;
accessing the internal portion of the bone through the aperture in the bone; and
applying the at least one loading condition to the internal portion of the bone, thereby facilitating structural changes in the bone supporting the articular surface”
Then he describes inserting the device into the bone:
“wherein the at least one loading condition is applied to the bone with a joint restoration system including a housing having an aperture therethrough, and an elongate member configured for insertion into the aperture in the housing, the method further comprising attaching the housing to the bone such that the aperture in the housing is generally aligned with the aperture in the bone, and
wherein applying the at least one loading condition to the internal portion of the bone includes inserting the elongate member through the apertures such that the elongate member contacts the internal portion of the bone and applies a force thereto.”
“the joint restoration system further including a compression member configured to cooperate with the housing to apply a force to the elongate member, the method further comprising inserting the compression member into the housing such that it contacts the elongate member and imparts a force thereto, thereby facilitating the application of the force to the internal portion of the bone by the elongate member.”
Here he describes the bone lengthening method:
“The method of claim 1, wherein the at least one loading condition is applied to an external portion of the bone such that the certain structural change includes at least one of an increase in a length of the bone or a decrease in a length of the bone.”
” The method of claim 6, wherein the at least one loading condition is applied to the bone with a joint restoration system including an electromechanical actuator, the method further comprising:
attaching the actuator to the external portion of the bone; and operating the actuator to apply the at least one loading condition to the external portion of the bone.”
” piezoelectric devices will often have displacements in the 100’s of micrometers, which will not provide enough travel to effect desired bone growth in many patients. To overcome this limitation, the actuator is provided with a secondary movement mechanism. The secondary mechanism is configured to provide a ratcheting, positive lock that outwardly extends the extendable component by some discrete amount. This allows the application of a stepwise series of 100 micrometer piezoelectric adjustments, until a total bone displacement of 1-5 mm displacement is achieved.”
The paper A LINEAR ACTUATED TORSIONAL DEVICE TO REPLICATE CLINICALLY RELEVANT SPIRAL FRACTURES IN LONG BONES, describes one potential way a linear actuator can be applied to bone. Although the device therein does not seem to be applied along the longitudinal axis as suggested by the patent but rather on the top and bottom of the bone.
This paper describes the use of a linear actuator to move a nail in distraction osteogenesis. Note that in the patent above it specifically states there there is no osteomy required for this device to lengthen bone.