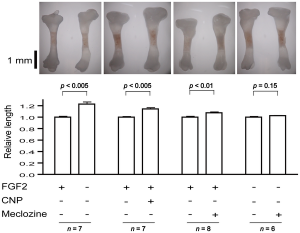
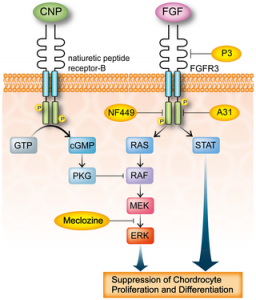
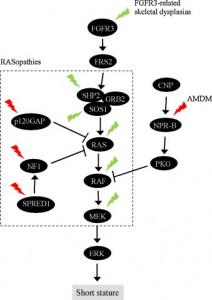
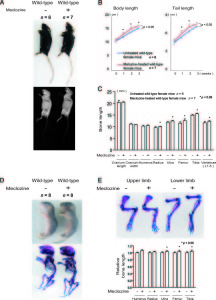
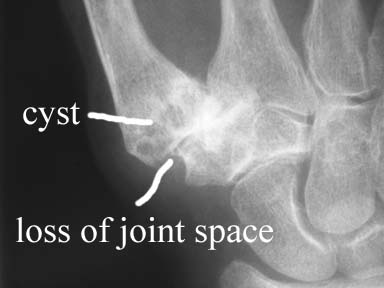
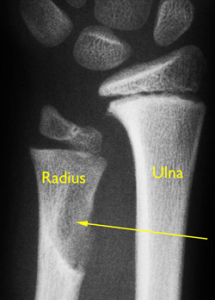
Growth plate regenaration has been covered before here and here. Ultimately, this provides more evidence that the presence of germ immature cells are the key limiting factor in creating new growth plates. Thus our primary focus should be on developing mechanical and supplemental methods that alter the structure of cells to be more like these growth plate chondrocyte precursor cells.
Regeneration of the Growth Plate
“The occurrence of growth plate regeneration has been doubted. However, in 5 different series of experiments reported between 1950 and 1986 regeneration of injured parts of growth plates in long bones of rabbits and pigs could be demonstrated. The 1st series implied partial X-ray injury of growth plates in rabbits aged 3–6 weeks.The 2nd series implied autotransplantation of the head of the fibula in rabbits aged 10–21 days. The 3rd, 4th and 5th series implied transplantation of autologous fat grafts into provoked defects of growth plates in rabbits and pigs. The findings show that regeneration of a growth plate occurs when a part of it is injured in such a manner that a bone bridge is not formed between the epiphysis and the metaphysis. Regeneration of a plate is much faster in relation to the growth in length of the bone in the rabbit than in the pig. The 1st and 2nd series suggest that regeneration takes place by interstitial proliferation of cells from the germinal layer of the uninjured parts of the plate. Signs of partial regeneration of growth plates have been seen in radiographs after operation for partial closure of growth plates in children. ”
Growth plate regeneration refers to the healing of damaged growth plates but if we learn the mechanisms by which growth plates are repaired we could potentially use those mechanisms to create new growth plates.
“Microscopic examination showed that injured portions of cartilage were pushed aside by the unirradiated cartilage growing into the irradiated area transversely in relation to the axis of the bone. In several experiments deformities and radiolucent foci appeared which showed similarity to those seen in dyschondroplasia{ectopic masses of cartilage}. When one half of the distal growth plate of the radius had been irradiated then lagging behind of ossitication could be demonstrated after 9 days.” Part of the injured cartilage was left behind in the metaphysis as the bone was growing. Another part adhered to the bony plate of epiphysis. In between, there was what appeared to be normal growing regenerating cartilage.
2nd series. Another set of experiments describes growth plate regeneration via interstitial growth. This type of growth would be very promising in terms of inducing adult height growth. No perichondrium and no zone of ranvier ossification groove was present. So there was no pool of typical growth plate progenitor cells.
“The regeneration seemed to take place from the viable parts of the germinal cell layer.”<-Bone and cartilage tends to come from the mesodermal layer. But mesodermal cells implies the type of immature cells like embryonic stem cells. But perhaps the right mechanical forces can still induce more adult mesenchymal stem cells into becoming more immature cells and into pre-chondrocyte growth plate cells.
“lt is generally accepted that interstitial latitudinal growth occurs in the very young animal, when the germinal zone cells of the growth plate are adjacent to a largely cartilaginous epiphysis as the tissues are plastic.”<-Plastic refers to irreversible deformation rather than elastic deformation where the object returns to normal. You can get plastic deformation in adult bones it’s just much harder.
Later the study states that interstitial growth can still occur even against a more rigid epiphysis as long as the germ cells are present.
Occurding to Figure 16 area C, the germinal cells look much like resting zone cells. We’ll have to study how much LSJL and other mechanical stimuli can induce mesenchymal stem cells to be more like mesodermal germinal cells.
A Mechanical Jack-like Mechanism Drives Spontaneous Fracture Healing in Neonatal Mice
“To study the mechanism underlying spontaneous regeneration of fractured bones, we left humeral fractures induced in newborn mice unstabilized, and rapid realignment of initially angulated bones was seen. This realignment was surprisingly not mediated by bone remodeling, but instead involved substantial movement of the two fragments prior to callus ossification. Analysis of gene expression profiles, cell proliferation, and bone growth revealed the formation of a functional, bidirectional growth plate at the concave side of the fracture. This growth plate acts like a mechanical jack, generating opposing forces that straighten the two fragments. Finally, we show that muscle force is important in this process, as blocking muscle contraction disrupts growth plate formation, leading to premature callus ossification and failed reduction.”
“The ossification of soft callus bares similarities to the process of endochondral ossification during skeletogenesis”
“another type of growth plate known as synchondrosis{these do fuse though} is located between the bones of the skull base. The synchondroses exhibit a remarkably organized structure, as each consists of two mirror-image growth plates facing opposite directions. These growth plates are fed by a shared resting zone located between them. The formation of double layers of prehypertrophic and hypertrophic chondrocytes drives growth in opposite directions, leading to expansion of the skull volume”
“the growth plate must also be able to generate considerable forces. Indeed, studies in various animal models as well as in humans suggest that the different growth plates can generate forces that range from the equivalent of 40% to 200% of body weight”
“an active bidirectional growth plate is formed at the concave side of the callus to mediate bone growth. This finding strongly supports our hypothesis that bone growth by the bidirectional growth plate generates the force required for the movement of the two fracture fragments during reduction, similar to a “mechanical jack” mechanism.”
“Expression analysis of SRY box containing gene 9 (Sox9), Col2a1, and Col10a1 revealed that the absence of muscle contraction led to symmetric chondrogenesis and loss of the bidirectional growth plate organization”
“in the absence of muscle contraction the callus fails to organize and act as a growth plate and undergoes early ossification”
“all of the characteristics of an active growth plate exist in the callus at the concave side of the fracture site, including gene expression profiles, cell proliferation, and bone growth. We therefore argue that the growth plate in the callus serves not only for intermediate stabilization, but also to actively promote bone reduction. However, unlike the epiphyseal growth plates and similar to the synchondroses that mediate cranial base expansion, the bidirectional growth plate at the fracture site drives growth in opposite directions. This generates force that moves the fragments toward straightening in a mechanical jack-like effect.”