I wrote about Resveratrol before and it is available for sale.
“Osteoporosis is a major public health issue that is expected to rise as the global population ages. Resveratrol (RES) is a plant polyphenol with various anti-aging properties. RES treatment of bone cells results in protective effects, but dose translation from in vitro studies to clinically relevant doses is limited since bioavailability is not taken into account. The aims of this review is to evaluate in vivo evidence for a role of RES supplementation in promoting bone health to reduced osteoporosis risk and potential mechanisms of action. Due to multiple actions on both osteoblasts and osteoclasts, RES has potential to attenuate bone loss resulting from different etiologies and pathologies. Several animal models have investigated the bone protective effects of RES supplementation. Ovariectomized rodent models of rapid bone loss due to estrogen-deficiency reported that RES supplementation improved bone mass and trabecular bone without stimulating other estrogen-sensitive tissues. RES supplementation prior to age-related bone loss was beneficial. The hindlimb unloaded rat model used to investigate bone loss due to mechanical unloading showed RES supplementation attenuated bone loss in old rats, but had inconsistent bone effects in mature rats. In growing rodents, RES increased longitudinal bone growth, but had no other effects on bone. In the absence of human clinical trials, evidence for a role of RES on bone heath relies on evidence generated by animal studies.”
“Resveratrol (RES) is a polyphenolic (3,4’,5-trihydroxystilbene) compound naturally present in red wine and a variety of plant foods such as grapes, cranberries, and nuts”
” human bone marrow-derived MSC with RES increased gene expression of the key osteogenic transcription factors, Runx2 and Osterix. RES was also demonstrated in vitro to act on various signal transduction pathways. RES activated the estrogen-mediated extracellular signal-regulated kinase 1/2 (ERK) signaling pathway regulating osteoblast differentiation and proliferation. RES activated AMP-activated protein kinase (AMPK) which regulates osteoblast differentiation and inhibits bone resorption by acting as a negative regulator of RANKL. RES augmented Wnt signaling which stimulated osteoblastogenesis and bone formation. Treating human bone marrow-derived MSC with RES promoted differentiation of MSC towards osteoblasts by up-regulating Runx2 gene expression through the activation of Sirt1. Also, activation of Sirt1 by RES was shown to promote binding to PPARγ which repressed MSC differentiation into adipocytes. Additionally, RES suppresses osteoclastogenesis by acting through Sirt1 to bind to RANK which inhibited binding to RANKL”<-many of these processes should impact longitudinal bone growth as well.
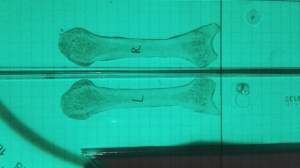
Resveratrol stimulated tibial and vertabral length in new zealand white rabbits that were 12 weeks old. 200mg per kg of bodyweight were given. Increased the amount of chondrocytes in the tibia and stimulated growth plate area while reducing fusion. Decreased vascularization indicated by lower VEGF and laminin levels.
“RES supplementation delayed growth plate fusion by suppressing the replacement of avascular cartilage with vascularized bone indicated by the down-regulated gene expression of vascular endothelial growth factor, a signaling molecule in vascularization, and laminin, a cartilage protein.”
In 6 month old Fisher-Brown Norway rats, it increased tibia length and width. Dosage was 12.5mg per kg of bodyweight.
“In vitro, RES treatment of chondrocytes obtained from an adult rat femur protected against the catabolic effect of pro-inflammatory cytokine, interleukin-1β”
“Osteoporosis is a major public health issue that is expected to rise as the global population ages. Resveratrol (RES) is a plant polyphenol with various anti-aging properties. RES treatment of bone cells results in protective effects, but dose translation from in vitro studies to clinically relevant doses is limited since bioavailability is not taken into account. The aims of this review is to evaluate in vivo evidence for a role of RES supplementation in promoting bone health to reduced osteoporosis risk and potential mechanisms of action. Due to multiple actions on both osteoblasts and osteoclasts, RES has potential to attenuate bone loss resulting from different etiologies and pathologies. Several animal models have investigated the bone protective effects of RES supplementation. Ovariectomized rodent models of rapid bone loss due to estrogen-deficiency reported that RES supplementation improved bone mass and trabecular bone without stimulating other estrogen-sensitive tissues. RES supplementation prior to age-related bone loss was beneficial. The hindlimb unloaded rat model used to investigate bone loss due to mechanical unloading showed RES supplementation attenuated bone loss in old rats, but had inconsistent bone effects in mature rats. In growing rodents, RES increased longitudinal bone growth, but had no other effects on bone. In the absence of human clinical trials, evidence for a role of RES on bone heath relies on evidence generated by animal studies. A better understanding of efficacy, safety, and molecular mechanisms of RES on bone will contribute to the determination of dietary recommendations and therapies to reduce osteoporosis.”
“eriosteal cortical bone formation coupled with endosteal cortical bone resorption regulates cross-sectional bone growth . To study bone growth, weanling female Sprague–Dawley rats were randomly assigned to a daily oral gavage of 0, 1, 4, 10, 40, 100 μg/d RES or estradiol (100 μg/d) dissolved in ethanol for a duration of 6 d. Despite estrogenic activity, RES had no significant effect on tibia cross-sectional area, medullary area, cortical bone area, periosteal bone formation rate or periosteal mineral apposition rat”
“In vitro, RES treatment of chondrocytes obtained from an adult rat femur protected against the catabolic effect of pro-inflammatory cytokine, interleukin-1B. To determine the effects of RES on longitudinal growth in vivo, a daily oral gavage of 200 mg/kg bwt RES [was given] to pubertal female New Zealand white rabbits until growth plate fusion occurred. After 16 weeks, rabbits provided RES supplementation had longer tibia and vertebrae, more chondrocytes, and increased growth plate area compared to control rabbits”
” Resveratrol, a natural polyphenol compound, may have the potential to promote bone formation and reduce bone resorption.”
“Resveratrol supplementation in the early ageing rats tended to decrease trabecular bone volume, Sirt1 gene expression and increased expression of adipogenesis-related genes in bone, all of which were statistically insignificant.”<- we want to increase sirt 1 levels.
“resveratrol supplementation does not significantly affect bone volume during the rapid growth phase but may potentially have negative effects on male skeleton during early ageing.”
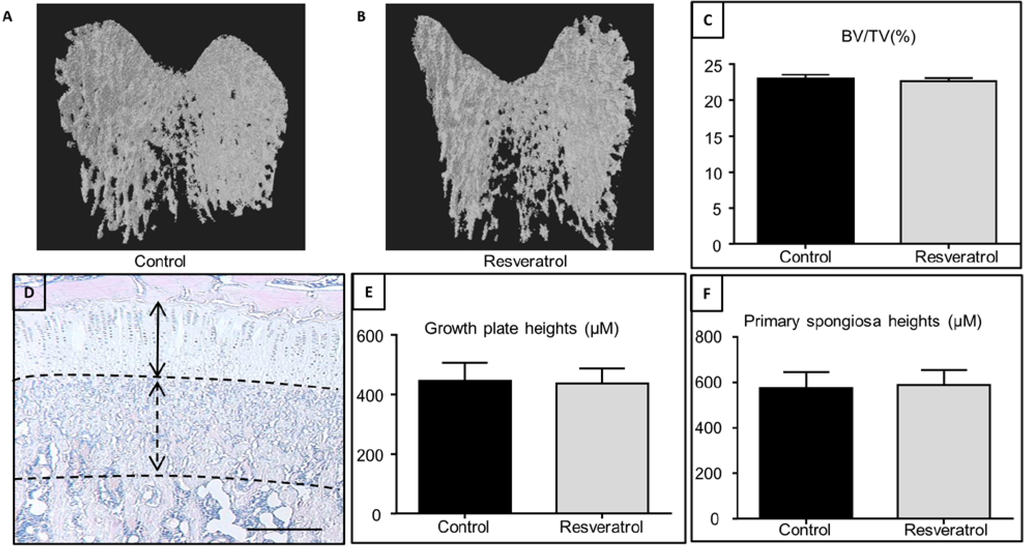
In this study resveratrol did not impact growth plate height.
“Resveratrol has been identified as a potent activator of Sirtuin 1 (Sirt1), which is also known as nicotinamide adenine dinucleotide (NAD)-dependent deacetylase”
“resveratrol supplementation for 5 weeks during the rapid growth period in male rats had no significant effects on growth plate thickness, primary spongiosa heights and trabecular bone volume by the end of treatment, suggesting a lack of effect of resveratrol supplementation on the bone mass outcome in growing rats. These findings are in agreement with a previous study using an isoflavone-enriched diet containing soybean extract, daidzein, genistein, and equol in 6-week-old growing female pigs, which also found no significant changes in the growth plate, mineralization or osteoblast/osteoclast densities in long bones”
Resveratrol inhibits adipocyte differentiation and cellular senescence of human bone marrow stromal stem cells
“Resveratrol up-regulates osteogenic differentiation of hBMSCs, it may contribute to protection against bone loss. Resveratrol targets senescence, oxidative stress and up regulates endogenous protective anti-oxidant pathway in hBMSCs.”<-reversing senescence may keep things like the growth plates open for longer.
“Resveratrol (RSV), a plant-derived antioxidant mediating biological effects via sirtuin- related mechanisms”
“RSV reduced the levels of senescence-associated secretory phenotype (SASP), gene markers associated with senescence (P53, P16, and P21), intracellular ROS levels and increased gene expression of enzymes protecting cells from oxidative damage (HMOX1 and SOD3).”