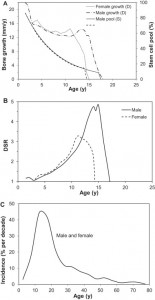
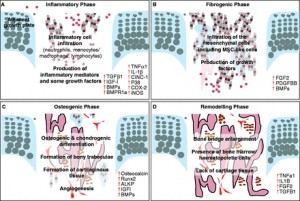
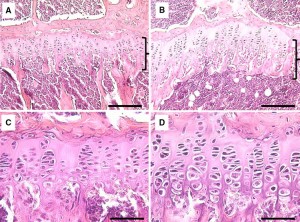
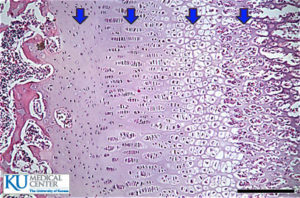
Unfortunately, not a lot of insight for LSJL for height growth as most of the effects are those related to the ability of joint loading to inhibit catabolism.
Mechanical intervention for maintenance of cartilage and bone.
“Moderate loads to the synovial joint suppress the expression levels of matrix metallproteinases (MMPs), while loads above a threshold tend to increase their destructive activities{although some catabolic effects of MMPs may be good for height growth, as MMPs may degrade bone allowing for cartilage growth}.“
“Moderate shear stress(2–5 dyn/cm2) reduced MMP expression levels, while high shear stress (10–20 dyn/cm2) increased them. Moderate hydrostatic pressure (1–5 MPa) suppressed MMP-1 expression, while higher loads (10 MPa) elevated it.”<-Since I have gotten more results with higher clamping force it could indicate that increased MMP expression is crucial to induce new length growth.
“The required magnitude of loads for joint loading is in general smaller than that for axial loading (e.g. 0.5 N for elbow loading and 2–3 N for ulna axial loading in mice). Bone is less stiff in a lateral direction than an axial direction.”<-Note that more than 0.5N(100N is mentioned) is likely required for humans. 0.5N is what was used in the mouse arm lengthening study.
“Joint loading periodically alters the pressure in the medullary cavity and activates molecular transport in a lacunocanalicular network in cortical bone.”<-It is our hypothesis that this increase in pressure in the medullary cavity induces chondrogenic differentiation. The medullary cavity is continuous into the spaces of the spongy bone of the epiphysis. It is these spaces where we aim to induce chondrogenic differentiation and thus induce endochondral ossification to grow taller.
“A pressure gradient in the medullary cavity generates oscillatory fluid flow in the porous bone cortex. Induced fluid flow then enhances molecular transport in the lacunocanalicular network and applies shear stress to osteocytes residing in lacunae”
“A pressure gradient in the medullary cavity generates oscillatory fluid flow in the porous bone cortex.”<-and fluid flow into the spongy bone spaces of the epiphysis and the increased MMP expression could allow the neo-growth plates to spread to other parts of the bone but this is highly speculative.
“Modulation of the intramedullary pressure with knee loading is exerted throughout the length of the tibia and the femur.”<-the epiphysis is part of the entire length thus knee loading like by LSJL alters pressure in the epiphysis.
“Proinflammatory cytokines such as IL-1β upregulate the expression and activity of MMP-1 and MMP-13. [In] cultured chondrocytes mechanical stimulation, given in a form of fluid flow shear stress, can suppress the IL-1β-induced upregulation of MMP-1 and MMP-13. In accordance with those in vitro results, joint motion in vivo is able to reduce inflammatory responses in a murine collagen-induced arthritis model. Additionally, in an antigen-induced arthritis model in rabbits, continuous passive motion suppressed transcription of IL-1β and synthesis of inflammatory mediator COX-2 and MMP-1. These mechanical signals also induced IL-10 synthesis, suggesting that moderate joint loading can generate anti-inflammatory signals.”<-MMP’s have complicated effects on height growth. MMP-1 and MMP-13 are vital for the endochondral ossificatiion process.
“When knee loading was applied to one leg, the loaded tibia and femur were reported to be longer than the non-loaded contralateral bones. In response to knee loading, the number of cells in the growth plate of the proximal tibia increased and their cellular shape was altered.“<-If LSJL increases the number of cells in the growth plate by differentiation of stem cells into chondrocytes than LSJL will work in adults as well. It’s possible that during knee loading only chondrocyte proliferation was increased but chondrocytes have a finite proliferative capacity and an increase in chondrocyte proliferation without increasing stem cell differentiation into chondrocytes should accelerate the the transition of proliferating chondrocytes into hypertrophic chondrocytes and not the number of cells in the growth plate.
“Homeostasis of the articular cartilage is affected through interactions with the subchondral bone underneath the cartilage. Both MMPs and ADAMTS need to be post-translationally activated, and this activation process is regulated by many factors including MMPs themselves and many proteoglycans.”<-Thus loading of the articular cartilage may itself play a role in the height gain by triggering a response in the subchondral bone in response to the stimulation of the articular cartilage. Thus underlying the importance of loading the synovial joint. The activation of MMPS and ADAMTS in the subchondral bone may play a role in neo growth plate formation.
“flexion of the joint in the presence of axial loads (5 N) increased the level of MMP-13 mRNA and its activity.”<-LSJL height growth can not be due to higher levels of MMP13 alone or axial loading would increase height!
“Whether mechanical loading can suppress or induce the integrated stress response is largely dependent on the loading intensity. This stress response leads to translational de-activation by a mechanism involving phosphorylation of eIF2α, with preferential translational activation of a particular set of proteins linked to cellular survival or apoptosis. In cultured chondrocytes, administration of thapsigargin and tunicamycin induces stress to the endoplasmic reticulum, which triggers an integrated stress response. In this response, the level of phosphorylated eIF2α was elevated together with the expression of MMP-13. Joint loading reduced the level of phosphorylated eIF2α by suppressing activity of Perk, one of the four known eIF2α kinases”