This study is important as although the scientists conclude that interstitial growth of bone is not possible(which would be a huge breakthrough for height growth) it provides a key term to describe the micro-growth plates that are the goal to be formed by LSJL(pseudoarthrosis). Although this term refers to a fracture case by definition it does show that a microgrowth plate can make a bone longer. The scientists suggest two possibilities as to how a bone can grow longer after spinal fusion: either institial growth of the bone itself or psuedoarthrosis(microgrowth plates). The scientists even state that microscopic areas of pseudoarthrosis may be be responsible for the lengthening in the conclusion.
Thus this study provides additional evidence that microgrowth plates can lengthen bone(as any amount of cartilage is more capable of interstitial growth(which increases bone length) unlike bone) and gives a new term to search for as a key to growing taller: pseudoarthrosis. The key is to find instances of psuedoarthrosis in adults that are spontaneous and not a result of surgery. If you want to help find a way to grow taller, helping find such studies would be a great boon.
Bone Growth after Spine Fusion A Clinical Survey
Full study is at the link below. It should be noted that this study is quite old but it’s still important today because now the focus is more on the gene expression rather than the mechanics as in the old days. And it’s easier for us to manipulate the mechanics rather than the gene expression.
bone growth after spinal fusion
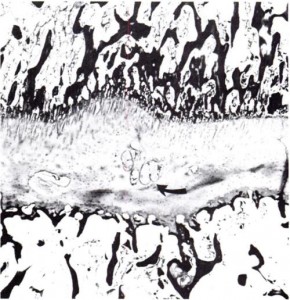
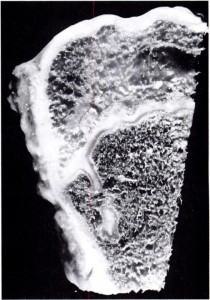
“When a spine fusion is unquestionably solid and fairly massive, there is little increase in length of the fused area. The small increase that most of the cases we studied showed could be accounted for by magnification and other technical factors, but it is impossible to rule out a small amount of growth. The slight decrease in the kyphos in two of our cases suggests some bending of the fusion mass. No definite pseudarthroses could be seen on the roentgenograms but the presence of one or more pseudarthroses could not be ruled out. Microscopic and transient pseudarthroses are considered by us to be the most likely mechanism by which any real increase in length or any true change in angulation occurs. In our experimental studies, microscopic losses of bone continuity in transepiphyseal bone grafts in the distal femora of young rabbits were demonstrated. These defects were of such a nature that they could not be demonstrated by standard clinical or roentgenographic methods.
In our opinion, the end-result study of Hallock and his associates is valuable in that it indicates, from the practical, clinical point of view, what will happen to the average patient after spine fusion in early childhood. However, their data and the observations of the other authors previously mentioned convey an inaccurate and perhaps unintentional impression that considerable growth occurs in a solidly fused spine segment. It would be unfortunate, we believe, to allow this impression to persist since surgeons not familiar with all that is known about growth of the spine after spine fusion might be falsely encouraged on the basis of published data to perform longer and more massive spine fusions in young children. Our study suggests that a long, massive, and completely solid fusion in early childhood will impair spine growth to a significant degree.
We believe that growth of a fused segment of the spine can occur only at the ends of the segment or at the site of gross or microscopic defects in the fusion plate. Pseudarthroses in spine fusions in children are much more frequent, in our opinion, than is generally suspected because of the tension forces exerted by the growing epiphyseal cartilages, as well as the usual stresses caused by motion. These pseudarthroses or stress fractures may be microscopic or grossly visible; they may occur spontaneously at any time and heal spontaneously. The more massive the fusion plate, the less chance there will be that it will break down under the stress of growth and motion. Finally, we believe that the laws that govern bone growth in general apply to the bone of spine fusions. There is in our opinion no such thing as interstitial growth of bone.”
“all growth in [bone] length of the diaphyses of long boneses takes place at the epiphyseal cartilages, whereas growth of bones its other dimensions occurs through hyaline-cartilage proliferation as in the epiphysis or through fibrous-tissue proliferation-as in the periosteum and flat bones.”
Scientists reported the apparent growth of fused spinal bones. If this were to happen in the solidly fused spine two conclusions could be made: either the fusion plate broken down or there was interstitial growth of bone in the fusion plate.
Scientists also observed that when interbody fusion was performed on the spine. The bony bridge that was formed elongated in response to vertebral growth. However, another study found that the fused area remained firm and did not increase in length in response to overall vertebral lengthening.
Another study with spinal fusion found that psuedoarthrosis occured at any interspace[Small hole surronded by bone]. An example of the interspace is perhaps the trabeculae. Pseudoarthrosis occurs at fracture areas. The scientists theorized that in their study the longitudinal bone growth was due to these areas of pseudoarhtrosis.
The study mentions Sincher’s law which states that: “increase of pressure or tension beyond the limits of tolerance leads to destruction of bone by resorption.”<-Perhaps LSJL needs to cause this destruction of bone in order to allow for microgrowth plates or psuedoarthrosis and that’s why so far LSJL on the finger has been more successful due to greater ability to increase pressure.