Here’s the before progress with the initial pic of the finger. Here’s the last pic of the finger
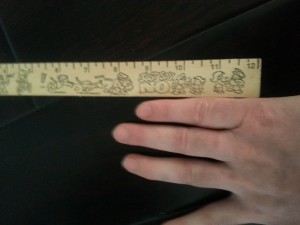
You can see a comparison of the left and right fingers and see that the right finger is longer than the left finger and much thicker.
Here’s a side to side comparison of the left and right fingers:
You’ll notice that the right finger is longer than the left finger and not that the right knuckle is higher than the left knuckle so there’s no measurement shennanigans. Is this pic enough to push as proof that LSJL finger growth is a success? Let me know if you’d suggest any adjustments.
Here’s another image:
Both the right finger is longer than the left finger at the tip and the right knucle is higher than the left knuckle. Nails on the bother inner most fingers are trimmed.
Here’s some pictures of my right hand from four years ago. Note that the method is obsolete but you can see a finger length increase between then and today.
Here’s a video with the right hand as well:
I found this video about performing LSJL:
http://youtu.be/sWlxaXfUeqM
This individual got results after 20 days which is likely due to the superior clamping device. And is more in line with the finger results I’ve gotten. I’ve gained about a 1/4 of inch in finger length and a drastic increase in finger width. Comparing the thumb pics it looks like there may be some length gain there but the thumb is farther along the ruler in the before pic but a side to side comparison of right to left thumb reveals that a possible increase of 1/8th of an inch or so.
So it’s been about 1 3/4 year loading my finger and I’ve gained say 1/4″. Since my finger is around 3 1/2″ long that’s about a 7% increase. Now a 7% increase in the tibia bone of say 16 inches would be 17.02 inches which would be a full inch of height. Now other than the initial gain in height at the beginning I have not gained significantly over time.
Now the key thing is despite my initial gain I’m not gaining length as rapidly in the legs as in the fingers and I’m not getting the drastic increase in width in the legs as in the fingers. This could be due to physiological differences between the leg and finger bones. The finger bones do not have periosteum for example or it could be due to the existing clamping device not being sufficient enough load. The load I’ve been using for 500 counts on the finger is quite extreme relative load.
I’ve been tweaking the load of the legs. Changing clamp position and bending the knee while loading the knee to get more leverage but we need to find a way to get the same kind of loads that can be done with the finger.
Importantly, we have finger proof. Longer fingers could be valuable to piano players and guitar players and possibly some other athletes. So how can we use this finger technique to raise funds to develop a more effective leg clamping device. I think we can go even further than this gentleman here in developing a better clamping device.
So:
What kind of evidence and documentation do I need to present to the layperson to show the finger proof of concept to generate interest and funding in developing a leg loading device?