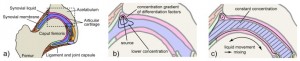
Regulation of growth plate and articular chondrocyte differentiation : implications for longitudinal bone growth and articular cartilage formation
“the embryonic Ihh/PTHrP feedback system is maintained postnatally except that the source of PTHrP has shifted to a more proximal location in the resting zone.”
“in articular cartilage, superficial chondrocytes differentiate from chondrocytes in the deeper layers following a program that has some similarities to the hypertrophic differentiation program in growth plate cartilage.”
“transplanting growth plate cartilage to the articular surface in an EGFP rat model that enabled cell tracing. We found that hypertrophic differentiation appeared to be inhibited in growth plate cartilage transplanted to the articular surface. The transplanted cartilage also underwent structural remodeling into articular-like cartilage, which suggests that the synovial microenvironment inhibits hypertrophic differentiation and promotes articular cartilage formation.”<-So if we change the bone microenvironment we could encourage growth cartilage.
“An essential genetic switch for patterning of skeletal elements is the expression of Hox genes, which encode a highly conserved family of transcription factors. Mesenchymal condensations are initially uninterrupted and at a later time differentiate into chondrocytes that express type II collagen”
“At the bottom of the proliferative zone, chondrocytes stop proliferating and undergo hypertrophy, a process characterized by gains in cell height, intracellular volume, and organelle size up to 4-, 10-, and 3-fold, respectively, and that also contributes to longitudinal bone growth”
“infection of embryonic chick limbs with retroviruses encoding BMP-2, -4, and GDF-5 increased chondrogenesis and final sizes of skeletal elements”
“in vitro administration of BMP-2 to rat fetal metatarsal bones or mouse embryonic stem cell lines increased chondrocyte proliferation and hypertrophy, whereas addition of Noggin elicited the opposite effect of preventing hypertrophic differentiation, thus indicating endogenous production of BMPs”
“mice deficient in both BMPR-IA and -IB receptors in cartilage lacked most skeletal elements that form by endochondral ossification and those that formed were rudimentary, demonstrating the importance of BMP signaling in early chondrogenesis. Conversely, mice overexpressing the BMPR-IA receptor had shortened proliferative columns and accelerated hypertrophic differentiation in the growth plate, suggesting BMP signaling also stimulates chondrocyte maturation”
“loss of BMP antagonism[BMP inhibitors] in Noggin and Gremlin knock-out mice led to multiple skeletal abnormalities including enlarged growth plates and defective patterning and outgrowth of limbs.”<-So increasing BMP expression does always increase growth but it depends on whether it’s worth the side effects.
“cartilage-specific overexpression of antagonist Smad6 in mice caused significantly delayed chondrocyte hypertrophy, thin trabecular bones, and dwarfism”
“activating mutations of PTHR1 cause Jansen’s metaphyseal chondrodysplasia characterized by short bowed limbs with normal growth plates but disorganized metaphyseal regions, whereas inactivating mutations of PTHR1 cause Blomstrand lethal chondrodysplasia characterized by short limbs, increased bone density, and advanced skeletal maturation”
“As proliferative chondrocytes grow distant from the source of PTHrP they undergo hypertrophy. Ihh is produced by prehypertrophic and hypertrophic chondrocytes and signals by perichondrium dependent and independent pathways to periarticular chondrocytes to express PTHrP, proliferative chondrocytes to increase the rate of cell division, and perichondrial cells to form bone collar”
“Perichondrial cells produce FGF-1, -2, -6, -7, -9, -18, -21, and -22, whereas growth plate chondrocytes only express FGF-2, -7, -18, and -22 at very levels, suggesting that FGFs from the perichondrium are the main regulators of chondrogenesis”
“In the growth plate and surrounding tissues, FGFR1 is expressed by prehypertrophic and hypertrophic chondrocytes, FGFR2 is expressed by the perichondrium and the c isoform by resting chondrocytes, FGFR3 expression has been more controversial being suggested in all zones, and FGFR4 is expressed by resting and proliferative chondrocytes”
“overexpression [of FGF-2] in mice causes shortened body length, expanded resting and
proliferative zones, and reduced hypertrophic zone”
“overexpression of Notch-1 in the ATDC5 chondrogenic cell line inhibited chondrogenesis and expression of Notch markers were shown to decline with the differentiation of human articular chondrocytes in pellet mass cultures”
“decreased Wnt signaling in articular cartilage compared to the growth plate due to
increased expression of Wnt antagonists FRP and Dkk-1″<-inhibitors of Wnt signaling may downregulate hypertrophic differentiation.
“We first found that Ihh, Patched, Smoothened, Gli1, Gli2, Gli3, and PTHR1 were expressed in regions analogous to the expression domains in prenatal epiphyseal
cartilage: Ihh was differentially expressed in the prehypertrophic (pre-HZ) and
hypertrophic (HZ) zones; Patched, the receptor for Ihh, was expressed in the resting
(RZ) and proliferative (PZ) zones and perichondrium; Smoothened, a second messenger
of Ihh signaling, was differentially expressed in RZ, PZ, and perichondrium; Gli1, Gli2,
and Gli3, transcription factors with activity downstream of Ihh, were differentially
expressed in RZ, PZ, and perichondrium; and PTHR1, the receptor for PTHrP, was
differentially expressed in pre-HZ and HZ. Most notable, however, was that PTHrP
was differentially expressed in RZ, which is a site that differs from the prenatal source
of PTHrP, the periarticular cells”
“We found that, at gestational day 16 (E16), lacZ activity was most pronounced in the superficial articular cartilage and perichondrium and gradually dissipated toward a minimum in HZ. At 1 week of age, lacZ activity was high in the articular cartilage, RZ, PZ, and perichondrium, whereas expression was low in HZ and minimal in hypertrophic cells located in the middle of the epiphysis where the secondary ossification later forms. At 4, 8, and 12 weeks of age, the lacZ activity pattern established at 1 week of age was largely maintained with distinct expression in the articular cartilage, RZ, PZ, and perichondrium, except that the superficial chondrocytes in articular cartilage lost detectable lacZ activity.”
“the prenatal Ihh/PTHrP feedback loop is maintained in the postnatal growth plate, except that the source of PTHrP has shifted to the resting zone. Since the number of resting zone chondrocytes decline with age[which produces PTHrP], our finding may explain why the height of proliferative columns shortens with age until the entire growth plate disappears at the end of puberty.”
Characterization of the proliferating layer chondrocytes of growth plate for cartilage regeneration.
“Cell-based therapy is a strategy capable of repair defect cartilage. At present, the articular chodrocytes (ACs) is the cell source for cartilage repair. Problematically, as serial culture, the ACs de-differentiation occurs, may result in graft failure. In present study, we evaluate the chondrogenic capacity and physical characteristics of proliferating layer chondrocytes (PLCs) derivates from growth plate cartilage, clarify its potential capacity for cartilage repair. We found that PLCs preserved more chrondrogenic phenotypes, such as polygonal appearances, whereas ACs appeared fibroblast-like after seventh passage{so growth plate chondrocytes have more differentiation ability}. Profoundly, the ACs expressed higher apoptosis-related proteins, such as cleaved-caspase-9 and cleaved-caspase-3, than PLCs. Also, the PLCs have higher proliferation rate than ACs, the cell-doubling time is 20.9h for PLCs, and 29.5h for ACs. Using flow cytometry, we demonstrated that 26.6% PLCs entered S-phase after 16h serum re-addition to starved cells, compared to 13.3% of ACs. Otherwise, col2a1, aggrecan, sox5, sox6 and sox9 mRNA were significantly increased in PLCs compared to ACs, in contrast, the col1a and col10a1 mRNA expression level in PLCs is less than in ACs. The glycosaminoglycan (GAG) content in PLCs was higher than ACs by the direct 1,9-dimethylmethylene blue (DMB) assay. Histological and immunohistochemical evaluations have demonstrated that significantly more chondrogenic extracellular matrix was detected in PLCs group when compared with ACs group after implantation in nude mice. Taken together, our data indicate the PLCs preserved much more chondrogenic phenotypes than ACs in vitro and in vivo. Those might imply that PLCs as the better cell source for transplantation can effectively repair and regenerate growth plate and articular cartilage.”