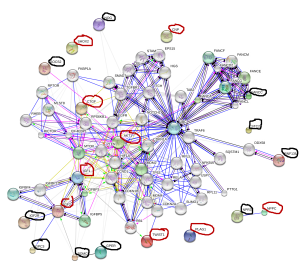
Click on the image for a better view. IGF2 is a strong candidate for increasing height including adults giving it’s role for inspiring progenitor cells that form new growth plates. The problem is how to increase it.
Target genes for height increase are as follows, for KO genes supplements or activities that inhibit these genes will increase height. For OE genes supplements or activities that upregulate these genes will increase height. For biphasic genes both overexpression and knockout decrease height. There are many biphasic genes but they are not listed.
KO(inhibit these)(Black):
FANCC
NOG
NPR3
RNF135
SOCS2
STC2
GPC3
IGF2R
GPR30
POMC
OE(stimulate these)(Red):
NPPC
PLAG1
SHOX2
Twist1
CNP
IGF2
IGF1
Akt1
CTGF(CCN2)
Notice how close four genes are in proximity to IGF2: IGF2R, GPC3, POMC, GPER. NPPC has a direct relationship with NPR3 with NPPC increasing height and NPR3 decreasing height. IGF2 is a strong central locus for increasing height also being connected to IGF1 and Akt1. LSJL increases Akt-phosphorylation by the way.
It turns out that the growth plate will regrow as long as their are progenitor cells, this area is called the zone of Ranvier. IGF2 could play a role in making these growth plate progenitor cells.
Since IGF2 is such a strong target for height growth how can we increase IGF2 levels for height?
Loss of imprinting of IGF2 and the epigenetic progenitor model of cancer.
“While IGF2 usually supports normal cellular growth, LOI of IGF2 may lead to overexpression of the gene and moreover global chromatin instability.”
“differentiated cells present in adult tissue that can acquire the ability to become undifferentiated and behave like stem cells, or progenitor cells”
” In contrast to mutations in the genetic sequence of DNA, epigenetic changes occur beyond the level of DNA and alter the protein-DNA complex that forms chromosomes. One such epigenetic factor is parental imprinting”
“Insulin-like growth factor 2 (IGF2), a gene whose end action is to stimulate general growth, is usually imprinted such that only the paternal allele is expressed. When LOI occurs, the maternal allele may also be expressed and some studies have, indeed, correlated LOI of IGF2 with increases in expression“<-So we can cause Loss of imprinting in adults to increase IGF2 expression in adults.
“The ICR for the IGF2/H19 locus is located in the 5’ flanking region of the H19 gene and 90kb downstream of IGF2. The ICR on the maternal allele is unmethylated, while the ICR on the paternal allele is methylated. This methylation of the paternal allele ICR blocks the transcription factor CCCTC-binding factor (CTCF) from binding and creating a physical barrier that stops downstream enhancers from augmenting IGF2 promoters. This effectively silences the maternal allele”
“Mouse models have shown that CTCF binds to regions near the IGF2 promoter, as well as the ICR, and subsequently forms CTCF-CTCF dimers, creating an intrachromosomal loop. The CTCF dimer then interacts with the SUZ12 (suppressor of zeste 12 homolog) domain of polycomb repressive complex 2 (PRC2) which methylates histone H3 lysine residue 27 (H3K27) causing silencing of the maternal allele. CTCF [synthesizes] decoy CTCF proteins. When introduced to cells, the decoys bind to the unmethylated ICR and IGF2 promoter but do not interact with SUZ12, thereby rendering Enhancer of zeste homolog 2 (EZH2), another part of PRC2, unable to methylate histone H3K27, resulting in reactivation of the imprinted allele{so the decoy CTCF are key to causing loss of imprinting of IGF2}. Neither intact CTCF sites nor hypermethylation at the ICR is sufficient for maintaining paternal allele silencing, and sequences outside of the CTCF binding sites at the ICR are needed for silencing”
“Thus, LOI of IGF2 may result from a variety of causes including: aberrant ICR methylation, a decreased expression of PRC2, a mutation of the ICR, or altered PRC2 H3K27 methylation. In contrast to the maternal allele, the paternal ICR is methylated, thereby blocking CTCF and PRC2 binding”
“A two-fold increase in IGF2 expression results in a 131% increase in offspring growth. Circulating IGF2 ligand has been shown to regulate crosstalk between the WNT and IGF1R pathways, which can lead to activation of either the phosphoinositide 3-kinase (PI3K)-AKT or the Ras-MAPK (mitogen-activated protein kinase) pathways that control metabolism, growth, differentiation, and apoptosis ”
“IGF2 expression levels thirty fold higher than wild type were not sufficient to develop tumors until senescence.”<-Elevated IGF2 levels do not directly cause cancer. Just more cells = more opportunities for malfunction=increased likelihood for cancer.
“LOI of IGF2 causes a bi-allelic expression of the gene, resulting in the overexpression of the IGF2 protein”
” a diet lacking synthetic methyl donors, including folic acid, vitamin B12, choline, and methionine, could cause LOI of IGF2 in murine models”<-Not really something you want to do however.
IGF2 injections were shown to induce height growth in an LSJL related study.
There are no known supplements that increase IGF2 in humans and I don’t know of any therapies involving injections.
Using CTCF decoy proteins may be a way though:
“Monoallelic expression of IGF2 is regulated by CCCTC binding factor (CTCF) binding to the imprinting control region (ICR) on the maternal allele, with subsequent formation of an intrachromosomal loop to the promoter region. The N-terminal domain of CTCF interacts with SUZ12, part of the polycomb repressive complex-2 (PRC2), to silence the maternal allele. We synthesized decoy CTCF proteins, fusing the CTCF deoxyribonucleic acid-binding zinc finger domain to CpG methyltransferase Sss1{not an easy thing to do homemade} or to enhanced green fluorescent protein. In normal human fibroblasts and breast cancer MCF7 cell lines, the CTCF decoy proteins bound to the unmethylated ICR and to the IGF2 promoter region but did not interact with SUZ12. EZH2, another part of PRC2, was unable to methylate histone H3-K27 in the IGF2 promoter region, resulting in reactivation of the imprinted allele. The intrachromosomal loop between the maternal ICR and the IGF2 promoters was not observed when IGF2 imprinting was lost. CTCF epigenetically governs allelic gene expression of IGF2 by orchestrating chromatin loop structures involving PRC2.”
” A maternally transmitted microdeletion of two CTCF binding sites in the ICR results in biallelic IGF2 expression and H19 silencing in Beckwith-Wiedemann syndrome”<-A syndrome that results in increased height.
Here’s a paper that states that Paxillin could be involved in IGF2 related growth:
Paxillin-dependent regulation of IGF2 and H19 gene cluster expression.
“Paxillin (PXN) is a focal adhesion protein that has been implicated in signal transduction from the extracellular matrix. Recently, it has been shown to shuttle between the cytoplasm and the nucleus. When inside the nucleus, paxillin promotes cell proliferation. Here, we introduce paxillin as a transcriptional regulator of IGF2 and H19 genes. It does not affect the allelic expression of the two genes; rather, it regulates long-range chromosomal interactions between the IGF2 or H19 promoter and a shared distal enhancer on an active allele. Specifically, paxillin stimulates the interaction between the enhancer and the IGF2 promoter, thus activating IGF2 gene transcription, whereas it restrains the interaction between the enhancer and the H19 promoter, downregulating the H19 gene. We found that paxillin interacts with cohesin and the mediator complex, which have been shown to mediate long-range chromosomal looping. We propose that these interactions occur at the IGF2 and H19 gene cluster and are involved in the formation of loops between the IGF2 and H19 promoters and the enhancer, and thus the expression of the corresponding genes. These observations contribute to a mechanistic explanation of the role of paxillin in proliferation and fetal development.”
“focal adhesion proteins can be found not only at focal adhesion contacts but also inside the nucleus and they can shuttle out of it and back in”
” The block of CRM1-dependent export pathway causes accumulation of paxillin inside the nucleus ”
“reactivation of IGF2 expression on the maternal allele has been previously reported in a number of human tumors and tumor cell lines”<-controlled reactivation could help us grow taller.
“SMC1A and MED23 play a role in paxillin-dependent regulation of the H19–IGF2 gene cluster. ”
An essential role for IGF2 in cartilage development and glucose metabolism during postnatal long bone growth.
“Postnatal bone growth involves a dramatic increase in length and girth. Intriguingly, this period of growth is independent of growth hormone and the underlying mechanism is poorly understood. Recently, an IGF2 mutation was identified in humans with early postnatal growth restriction. Here, we show that IGF2 is essential for longitudinal and appositional murine postnatal bone development, which involves proper timing of chondrocyte maturation and perichondrial cell differentiation and survival. Importantly, the Igf2 null mouse model does not represent a simple delay of growth but instead uncoordinated growth plate development. Furthermore, biochemical and two-photon imaging analyses identified elevated and imbalanced glucose metabolism in the Igf2 null mouse. Attenuation of glycolysis rescued the mutant phenotype of premature cartilage maturation, thereby indicating that IGF2 controls bone growth by regulating glucose metabolism in chondrocytes. This work links glucose metabolism with cartilage development and provides insight into the fundamental understanding of human growth abnormalities.”
“Newly formed chondrocytes are proliferative and morphologically round, but eventually become flat chondrocytes to form the ‘columnar zone’”
“the Igf2 null growth plate cartilage was shorter and disproportionally thinner than the WT. The mutant growth plate was generally well formed, but its hypertrophic zone was disproportionally larger and the epiphyseal zone shorter. In addition, there was a clear delay in SOC formation in the mutant, which contributed to the shortened cartilage template ”
“IGF2 deficiency caused a shortening of the prehypertrophic zone ”
” Our prior study on human adult articular chondrocytes also failed to detect Akt activation by IGF2, suggesting that IGF2 may act differently than IGF1 in chondrocytes ”
“the regulatory role of IGF2 on chondrocyte maturation and matrix production in endochondral ossification is mediated by its activity on glucose metabolism in chondrocytes.”
“TheIgf1r null mouse has shorter bones, but the IR null mouse has a normal bone length. However, both knockouts had a reduced hypertrophic zone which is consistent with the phenotype of the Igf1 knockout. Igf2 null bones, on the other hand, exhibit a disproportionally larger hypertrophic zone. These data indicate different roles of IGF1 and IGF2 on cartilage development. Consistent with this notion, although IGF2 overexpression promoted postnatal growth, it failed to compensate the phenotype caused by the loss of IGF1 ”
“IGF2 is unique in its ability to bind to IGF2R, and it has been shown that the direct binding of IGF2 to IGF2R stimulated proteoglycan synthesis and induced calcium influx in chondrocytes, as it occurs even in the presence of an antiIGF-IR antibody.On the other hand, knockout of Igf2r exhibits increased skeletal growth{Maybe because this encourages IGF2 to bind to different receptors? Thus,none of the single knockout of the potential receptors exhibits the same phenotype as that of the Igf2 null mouse. However, it is possible that the phenotype of the Igf2 null mouse is a result of its binding to multiple receptors, together with interaction of multiple IGF-binding proteins and subsequent complex downstream signaling”