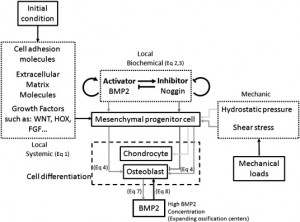
The generation of loads in excess of the osteogenic threshold by physical movement.
“stresses in bones in excess of the osteogenic threshold will stimulate bone growth; however, protocols for the generation of these stresses had not been established. Two trial movements were examined in the study: the plié and a movement requiring the subject to move a leg sequentially to 45° displaced positions – the star excursion balance test. Using inverse dynamics and an optimisation approach, the loads in the muscles crossing the hip and knee joints and the corresponding joint contact forces were calculated. It was found that the osteogenic threshold was exceeded in both these trials identifying them as suitable exercises in the maintenance of bone health. In the order of increasing bone load at the hip, and hence increasing bone growth stimulation, are the following demi plié, star excursion balance test with maximum reach criterion, grande plié and star excursion balance test with maximum speed criterion. In the order of increasing bone load at the knee are demi plié, grande plié, star excursion balance test with maximum reach criterion and star excursion balance test with maximum speed criterion. However, due to the high loads encountered, these exercises are not recommended for subjects with advanced osteoporosis although the boundary between therapeutic bone loading leading to increase in bone mineral density and loads capable of causing fracture is unclear.”
“BMD fracture thresholds:110 mg/cm^3 in the distal femur and 70 mg/cm^3 in the proximal tibia”
“threshold of stress at which bone growth is stimulated corresponding to a stress of approximately 20MPa, which for a bone’s Young’s modulus of 20 GPa is 1000me.”
“As the lever arm at which the muscles act at the joint is typically relatively small compared to the segment length to which they are attached, the loads in the muscles and hence the compressive forces in the joint and bone can be very large”
“during a quasi-static rise from a deep squat, the tibio-femoral joint forces were 5.5–6.6 times the body weight.”
“a hip joint contact force of 2.38 times body weight occurred while walking at 4 km/h, 2.51 times body weight during stair ascent and 2.60 times body weight during stair descent.”
The star excursiion balance test generated a force around 6 times bodyweight in the knee.