Here’s a study that shows that growth plate loading can stunt growth but it also shows that it can enhance growth in one vertebrae which emphasizes the importance of developing the proper loading method.
In vivo dynamic loading reduces bone growth without histomorphometric changes of the growth plate
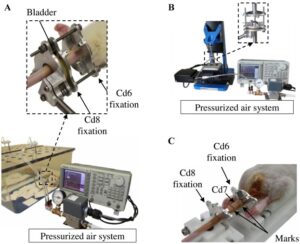
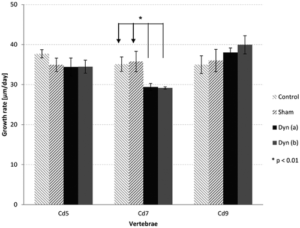
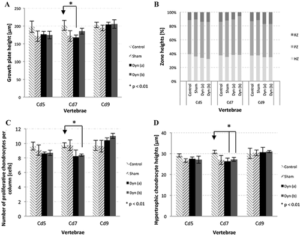
“This in vivo study aimed at investigating the effects of dynamic compression on the growth plate. Rats (28 days old) were divided into three dynamically loaded groups, compared with two groups (control, sham). A device was implanted on the 6th and 8th caudal vertebrae for 15 days{but note not on the 9th vertebrae which grows as we’ll see later}. Controls (n = 4) did not undergo surgery. Shams (n = 4) were operated but not loaded. Dynamic groups had sinusoidal compression with a mean value of 0.2 MPa: 1.0 Hz and ±0.06 MPa (group a, n = 4); 0.1 Hz and ±0.2 MPa (group b, n = 4); 1.0 Hz and ±0.14 MPa (group c, n = 3). Growth rates (µm/day) of dynamic groups (a) and (b) were lower than shams (p < 0.01). Growth plate heights, hypertrophic cell heights and proliferative cell counts per column did not change in dynamic (a) and (b) groups compared with shams (p > 0.01). Rats from dynamic group (c) had repeated inflammations damaging tissues{this group had the highest frequency of loading}; consequently, their analysis was unachievable. Increasing magnitude or frequency leads to growth reduction without histomorphometric changes. However, the combined augmentation of magnitude and frequency alter drastically growth plate integrity. Appropriate loading parameters could be leveraged for developing novel growth modulation implants to treat skeletal deformities{the authors themselves even allude to with this sentence that this does not disprove growth modulation but that it only suggests that it’s important to develop the appropriate growth modulation method}.”
“With the approval of the Institutional Animal Care Committee, 19 male Sprague–Dawley rats were received at the age of 21 days old. After 1-week of acclimatization, the protocol was conducted from 28 to 43 days old, corresponding to the rat pubertal growth spurt”
“The stress variation was chosen at a mean value of 0.2 MPa as it represents a wide physiological stress interval that is still retarding but not arresting bone growth (stress value over 0.6 MPa). The frequencies were either lower (0.1 Hz) or close to an average walking frequency (1.0 Hz) for humans. ”
“The loading device was adjusted daily at the same time of day for 10 min under isoflurane to compensate for longitudinal growth.”
“three caudal vertebrae (one loaded Cd7; two unloaded (Cd5, Cd9) used as intra-animal controls)”
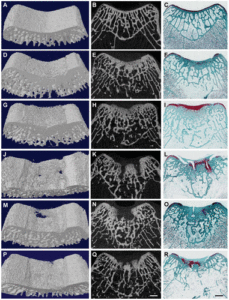
Note how the CD9 vertebrae grew significantly faster than controls. Also interestingly the sham group for both CD7 and CD9 vertebrae grew faster than controls. “Shams (n = 4) were operated but no compression was applied.” <-so the operation itself could be stimulatory on the growth
“The ultimate dynamic (c) condition, combining an increase in both maximum magnitude and frequency, was tested only on three animals since severe inflammation occurred.”
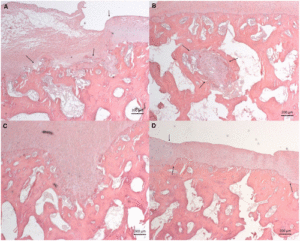
CD9 has a significant increase in all growth plate parameters according to these figures.
Clearly the loading regime on CD7 had spillover reduction in growth in CD5 and stimulatory effect on growth in CD9. It’s hard to say why that is without looking at say a stress strain analysis. The device could be doing indirect tensile strain on CD9 and compressive strain on CD5. But this study shows the importance of finding the right loading method in order to manipulate longitudinal bone growth.