There a few possibilities as to why the height gain occurs:
- Improvement in posture
- Increase in bone density reducing bone compression
- Longitudinal bone growth
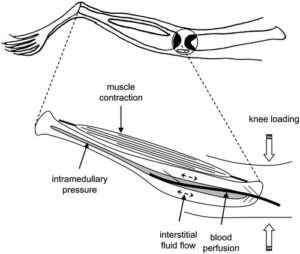
“Optimal osteogenic mechanical loading requires the application of high‐magnitude strains at high rates{I’m not sure this is the case given fluid flow models}. High‐intensity resistance and impact training (HiRIT) applies such loads but is not traditionally recommended for individuals with osteoporosis because of a perceived high risk of fracture. The purpose of the LIFTMOR trial was to determine the efficacy and to monitor adverse events of HiRIT to reduce parameters of risk for fracture in postmenopausal women with low bone mass. Postmenopausal women with low bone mass (T‐score < –1.0, screened for conditions and medications that influence bone and physical function) were recruited and randomized to either 8 months of twice‐weekly, 30‐minute, supervised HiRIT (5 sets of 5 repetitions, >85% 1 repetition maximum) or a home‐based, low‐intensity exercise program (CON). Pre‐ and post‐intervention testing included lumbar spine and proximal femur bone mineral density (BMD) and measures of functional performance (timed up‐and‐go, functional reach, 5 times sit‐to‐stand, back and leg strength). A total of 101 women (aged 65 ± 5 years, 161.8 ± 5.9 cm, 63.1 ± 10.4 kg) participated in the trial. HiRIT (n = 49) effects were superior to CON (n = 52) for lumbar spine (LS) BMD (2.9 ± 2.8% versus –1.2 ± 2.8%, p < 0.001), femoral neck (FN) BMD (0.3 ± 2.6% versus –1.9 ± 2.6%, p = 0.004), FN cortical thickness (13.6 ± 16.6% versus 6.3 ± 16.6%, p = 0.014), height (0.2 ± 0.5 cm versus –0.2 ± 0.5 cm, p = 0.004){0.2 cm is pretty significant. If the gain is due to posture then wouldn’t the low intensity exercise program gain posture as the low intensity group would also build functional strength?}, and all functional performance measures (p < 0.001). Compliance was high (HiRIT 92 ± 11%; CON 85 ± 24%) in both groups, with only one adverse event reported (HiRIT: minor lower back spasm, 2/70 missed training sessions). Our novel, brief HiRIT program enhances indices of bone strength and functional performance in postmenopausal women with low bone mass. Contrary to current opinion, HiRIT was efficacious and induced no adverse events under highly supervised conditions for our sample of otherwise healthy postmenopausal women with low to very low bone mass.”
One thing to consider is that there may be a load threshold for bone strengthening. Since the women were so weak 85% of 1RM was needed to get above the load but for a stronger individual a smaller percentage of the 1RM may theoretically be needed.
” Resistance exercises (deadlift, overhead press, and back squat) were performed for the remainder of the intervention period in 5 sets of 5 repetitions, maintaining an intensity of >80% to 85% 1 RM. Participants performed up to 2 sets of deadlifts at 50% to 70% of 1 RM to serve as a warm‐up, as required. Impact loading was applied via jumping chin‐ups with drop landings. Participants were instructed to grasp an overhead bar with their shoulders and elbows flexed to 90 degrees, and their hands shoulder width apart with an underhand grip. The participant then jumped as high as possible while simultaneously pulling themselves as high as possible with their arms. At the peak of the jump, the participant dropped to the floor, focusing on landing as heavily as comfortably possible. Each exercise session was performed in small groups with a maximum of 8 participants per instructor, who was an exercise scientist and physiotherapist.”
I’m not sure if impact loading would be superior to tapping. Tapping applies the load directly to the bone and whole body impact can damage soft tissue(although so can tapping). I feel it more in the bone when tapping than whole body impact.
“Participants allocated to CON undertook an 8‐month, twice‐weekly, 30‐minute, home‐based, low‐intensity (10 to 15 repetitions at <60% 1 RM) exercise program designed to improve balance and mobility but provide minimal stimulus to bone. The CON program consisted of walking for warm‐up (10 minutes) and cool down (5 minutes), low‐load resistance training (lunges, calf raises, standing forward raise, and shrugs) and stretches (side‐to‐side neck stretch, static calf stretch, shoulder stretch, and side‐to‐side lumbar spine stretch). The intensity of resistance exercises was progressed from body weight to a maximum of 3 kg hand weights for the final month of the program.”<-I think this exercise regime would improve posture. But if you look at table 2 this group lose 0.2cm of height. Although if you look at the back extensor strength increase in table 5 the amount is far more in the high intensity group which would look to posture.
“Although not originally a primary outcome measure, we observed that HiRIT improved stature compared with CON. The observed improvement in stature after HiRIT is likely to be a consequence of increased BES(back extensor strength), as BES is inversely associated with magnitude of kyphosis. Our results add support to the findings of other exercise intervention studies that have demonstrated improvements in BES by 21% and corresponding kyphosis reductions of 5° to 6° in postmenopausal hyperkyphotic women”
Now we don’t want the height gain to be due to back extensor strength. We want the height to be due to longitudinal bone growth.
According to Short-term and long-term site-specific effects of tennis playing on trabecular and cortical bone at the distal radius, “In children, no significant difference was observed between the dominant and nondominant forearm lengths (21.6 cm on both sides). In adults, the respective values were 25.3 ± 1.6 cm and 25.0 ± 1.6 cm, with a significant side-to-side difference”. So also a bone density increase correlates with about a .3cm gain and you can’t account for this gain in terms of posture.
More on tennis increasing bone length can be found here.
Whole-body morphological asymmetries in high-level female tennis players: A cross‑sectional study
“This cross-sectional study aimed to examine the degree of whole-body morphological asymmetries in female tennis players. Data were collected in 19 high-level female tennis players (21.3 ± 3.4 years). Based on anthropometric measurements (upper arm, lower arm, wrist, upper leg and lower leg circumferences as well as elbow and knee widths) and dual x-ray absorptiometry research scans (bone mineral density (BMD), bone mineral content (BMC), lean mass (LM), fat mass (FM) as well as humerus, radio-ulnar, femur and tibia bone lengths), within-subject morphological asymmetries for both upper (dominant vs. non-dominant) and lower (contralateral vs. ipsilateral) extremities were examined. Upper arm (p = 0.015), lower arm (p < 0.001) and wrist circumferences (p < 0.001), elbow width (p = 0.049), BMD (p < 0.001), BMC (p < 0.001), LM (p = 0.001), humerus (p = 0.003) and radio-ulnar bone length (p < 0.001) were all greater in the dominant upper extremity. BMC (p < 0.001) and LM (p < 0.001) were greater in the contralateral lower extremity, whereas FM (p = 0.028) was greater in the ipsilateral lower extremity.”
“Humerus bone length (cm) 27.6 ± 1.1(dominant)26.9 ± 1.2(non-dominant) Radio-ulnar bone length (cm) 25.0 ± 1.2(dominant)24.3 ± 1.3(non-dominant)”
I don’t think we can rule out that bone density can increase bone length. It’s possible that since gains are typically small 0.2cm-1cm that they aren’t considered. You’ll remember above that that the tennis study found the increase in bone length only in adult and not in children. I don’t know if whole body impact is the best just based on personal testing I think direct impact is the best. Tennis is pretty close to direct impact but the load is very inconsistent being based on how the game is going. It may be possible to get larger increases with direct impact. Whole body impact is skill dependent, runs risk of soft tissue injury, and you may land wrong. It’d be interesting if a bone density regime like osteostrong has any impact on bone length or height parameters.