This study basically explains that macrophages are key to neo-endochondral ossification:
Fracture healing via periosteal callus formation requires macrophages for both initiation and progression of early endochondral ossification.
“The distribution, phenotype, and requirement of macrophages for fracture-associated inflammation and/or early anabolic progression during endochondral callus formation were investigated. A murine femoral fracture model [internally fixed using a flexible plate (MouseFix)] was used to facilitate reproducible fracture reduction. IHC demonstrated that inflammatory macrophages (F4/80(+)Mac-2(+)) were localized with initiating chondrification centers and persisted within granulation tissue at the expanding soft callus front. They were also associated with key events during soft-to-hard callus transition. Resident macrophages (F4/80(+)Mac-2(neg)), including osteal macrophages, predominated in the maturing hard callus. Macrophage Fas-induced apoptosis transgenic mice were used to induce macrophage depletion in vivo in the femoral fracture model. Callus formation was completely abolished when macrophage depletion was initiated at the time of surgery and was significantly reduced when depletion was delayed to coincide with initiation of early anabolic phase. Treatment initiating 5 days after fracture with the pro-macrophage cytokine colony stimulating factor-1 significantly enhanced soft callus formation. The data support that inflammatory macrophages were required for initiation of fracture repair, whereas both inflammatory and resident macrophages promoted anabolic mechanisms during endochondral callus formation. Overall, macrophages make substantive and prolonged contributions to fracture healing and can be targeted as a therapeutic approach for enhancing repair mechanisms. Thus, macrophages represent a viable target for the development of pro-anabolic fracture treatments with a potentially broad therapeutic window.”
“inflammatory macrophages were required for initiation of fracture repair, whereas both inflammatory and resident macrophages promoted anabolic mechanisms during endochondral callus formation.”
“Periosteal endochondral callus formation progresses via four sequential and interdependent phases: inflammation leading to granulation tissue formation, early anabolism (soft cartilaginous callus formation), late anabolism (hard bony callus formation), and remodeling to reinstate the original bone architecture and mechanical strength”
“recruited inflammatory macrophages are derived from blood monocytes and rapidly infiltrate tissues compromised by injury, abnormal function, and/or infection”
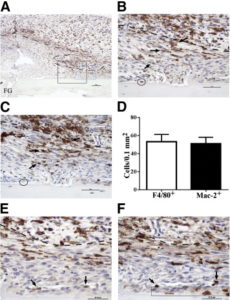
“Inflammatory macrophages predominate in fracture granulation tissue and associate with chondrification centers. Representative images of periosteal fracture zone (Supplemental Figure S1A) 7 days after osteotomy and MouseFix plate fixation surgery in 12-week-old C57Bl/6 mice (n = 6 with assessment at multiple sectional depths per sample). All sections were counterstained with hematoxylin. A: Image represents approximately half of the periosteal fracture zone (proximal) with the osteotomy-generated fracture gap (FG) at the bottom left of the image. Granulation tissue predominates at this time point. Tissue section was stained by IHC for the F4/80 pan-macrophage antigen (brown). The black box demarks the region shown in B and C. The blue box demarks the region shown in E and F. B: IHC for F4/80 expression within fracture granulation tissue. The dashed line demarks the interface between the mesenchymal (closest to bone, below dashed line) and inflammatory (above dashed line) stratum. F4/80+ cells with stellate morphological characteristics are evident in both the inflammatory and mesenchymal (arrows) strata. The circle demarks a single osteomac within this field. C: IHC for Mac-2 expression (brown) in a serial section to that shown in B. Arrows point to the same cells indicated in B and highlight the high degree of overlap in F4/80 (B) and Mac-2 (C) staining patterns. The circle indicates the Mac-2neg osteomac identified in B. D: Quantification of the number of F4/80+ and macrophage-like Mac-2+ cells within the mesenchymal stratum of the granulation tissue. An average area of 0.15 mm2 was assessed in six independent samples, and the number of positive cells was not statistically different. E: F4/80+ macrophages (brown, arrows) adjacent to a periosteal chondrification center. F: Mac-2 staining in a serial section to E confirms induction of Mac-2 expression in condensing chondrocyte-like cells within the periosteal chondrification center (blue boxed area). The same F4/80+ macrophages noted in E can be traced and express Mac-2 (arrows). Dashed line in B, C, E, and F demarks the mesenchymal (lower)- inflammation (upper) strata junction within the granulation tissue. Original magnifications: ×10 (A), ×40 (B and C); ×60 (E and F). Scale bars: 100 μm (A); 50 μm (B and C); 37.5 μm (E and F).”
“chondroblasts were identified as F4/80negMac-2+ condensed mesenchymal cells (Figure 1F). F4/80+Mac-2+ (Figure 1, E and F) inflammatory macrophages were observed adjacent to chondrification centers and associated vascular structures.”
” Cartilage and woven bone formations were absent within the periosteal fracture zone [with no macrophages]”
Macrophages are also present in the soft to hard callus transition although this is not as important for purposes as creating the initial growth plate is the limiting factor.
” inflammatory macrophages [were present] in the mesenchymal stratum of the granulation tissue, including some that were associated with developing chondrification centers”
“The developmental vascular canals were broad invaginations with osteoclasts/chondroclasts at the apical tip, presumably excavating the canal path via matrix degradation, followed by a mixture of mesenchymal cells, osteoclasts/chondroclasts, lysosomal cells, macrophages, and endothelial cells.”
“macrophages are pro-mitogenic toward chondrocytes.”<-macrophages undergo chondrocytes to undergo cell division.
Resting and injury-induced inflamed periosteum contain multiple macrophage subsets that are located at sites of bone growth and regeneration.
“Better understanding of bone growth and regeneration mechanisms within periosteal tissues will improve understanding of bone physiology and pathology. Macrophage contributions to bone biology and repair have been established but specific investigation of periosteal macrophages has not been undertaken. We used an immunohistochemistry approach to characterise macrophages in growing murine bone and within activated periosteum induced in a mouse model of bone injury. Osteal tissue macrophages (osteomacs) and resident macrophages were distributed throughout resting periosteum. Tissues were collected from 4 week old mice and osteomacs were observed intimately associated with sites of periosteal diaphyseal and metaphyseal bone dynamics associated with normal growth. This included F4/80+Mac-2-/low osteomac association with extended tracks of bone formation (modeling) on diphyseal periosteal surfaces. While this recapitulated endosteal osteomac characteristics, there was subtle variance in the morphology and spatial organization of modelling-associated osteomacs, which likely reflects the greater structural complexity of periosteum. We also demonstrated that osteomacs, resident macrophages and inflammatory macrophages (F4/80+Mac-2hi) were associated with the complex bone dynamics occurring within the periosteum at the metaphyseal corticalization zone. These 3 macrophage subsets were also present within activated native periosteum after bone injury across a 9 day time course that spanned the inflammatory through remodeling bone healing phases. This included osteomac association with foci of endochondral ossification within the activated native periosteum. These observations confirm that osteomacs are key components of both osteal tissues, in spite of salient differences between endosteal and periosteal structure and that multiple macrophage subsets are involved in periosteal bone dynamics”
“The periosteum is a specialized connective tissue composed of a vascularized and innervated fibrous membrane that encapsulates bone. It has two layers: an outer fibrous capsule layer containing elastic connective tissue (including Sharpey’s fibres), fibroblasts and blood vessels; and, an inner cambium layer containing capillaries, nerves, pre-osteoblasts/bone lining cells, osteoblasts and undifferentiated mesenchymal stromal/stem cells (also referred to as periosteum-derived progenitor cells)”<-these stem cells could potentially be involved in neo growth plate formation.
“Progenitor cells within the endosteum and periosteum have different potential: endosteal progenitors are restricted to osteoblastic differentiation but periosteal progenitors have osteoblastic and chondrocytic bi-potential.”<-It isn’t necessarily true that endosteal progenitors are restricted to osteoblast differentiation it could be influenced by the microenvironment.
Macrophages in bone fracture healing: Their essential role in endochondral ossification.
“In fracture healing, skeletal and immune system are closely interacting through common cell precursors and molecular mediators. It is thought that the initial inflammatory reaction, which involves migration of macrophages into the fracture area, has a major impact on the long term outcome of bone repair. Interestingly, macrophages reside during all stages of fracture healing. Thus, we hypothesized a critical role for macrophages in the subsequent phases of bone regeneration. This study examined the impact of in vivo induced macrophage reduction, using clodronate liposomes, on the different healing phases of bone repair in a murine model of a standard closed femoral fracture. A reduction in macrophages had no obvious effect on the early fracture healing phase, but resulted in a delayed hard callus formation, thus severely altering endochondral ossification. Clodronate treated animals clearly showed delayed bony consolidation of cartilage and enhanced periosteal bone formation. Therefore, we decided to backtrack macrophage distribution during fracture healing in non-treated mice, focusing on the identification of the M1 and M2 subsets. We observed that M2 macrophages were clearly prevalent during the ossification phase. Therefore enhancement of M2 phenotype in macrophages was investigated as a way to further bone healing. Induction of M2 macrophages through interleukin 4 and 13 significantly enhanced bone formation during the 3week investigation period. These cumulative data illustrate their so far unreported highly important role in endochondral ossification and the necessity of a fine balance in M1/M2 macrophage function, which appears mandatory to fracture healing and successful regeneration.”
The failure of endochondral ossification was macrophage specific and not indirectly related to osteoclasts. Osteoclasts and macrophages come from the same progenitor cell.