Hydrostatic pressure is a force we can manipulate via mechanical stimulation.
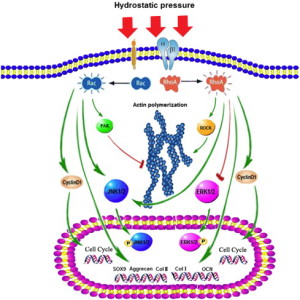
Hydrostatic pressure promotes the proliferation and osteogenic/chondrogenic differentiation of mesenchymal stem cells: The roles of RhoA and Rac1.
“Hydrostatic pressure can serve as an active regulator for bone marrow mesenchymal stem cells (BMSCs). [We investigate the roles] of cytoskeletal regulatory proteins Ras homolog gene family member A (RhoA) and Ras-related C3 botulinum toxin substrate 1 (Rac1) in hydrostatic pressure-related effects on BMSCs. Hydrostatic pressure promoted cell cycle initiation in a RhoA- and Rac1-dependent manner. RhoA played a positive and Rac1 displayed a negative role in the hydrostatic pressure-induced F-actin stress fiber assembly. RhoA and Rac1 play central roles in the pressure-inhibited ERK phosphorylation, and Rac1 but not RhoA was involved in the pressure-promoted JNK phosphorylation. Pressure promoted the expression of osteogenic marker genes in BMSCs at an early stage of osteogenic differentiation through the up-regulation of RhoA activity. Pressure enhanced the expression of chondrogenic marker genes in BMSCs during chondrogenic differentiation via the up-regulation of Rac1 activity. RhoA and Rac1 are critical to the pressure-induced proliferation and differentiation, the stress fiber assembly, and MAPK activation in BMSCs.”
“Hydrostatic pressure applications are methods of applying mechanical loading that mimics the compressive forces borne by cartilage in a joint cavity“<-So we should be able to apply such mechanical load ourselves but in the bone rather than the cartilage to encourage a neo-ectopic growth plate.
“the activities of Rho GTPase signaling molecules are closely related to the differentiation and the fate of BMSCs.”
“the activation of the RhoA pathway and the inhibition of the Rac1 pathway under hydrostatic pressure promoted the assembly of the F-actin cytoskeleton, whereas the inhibition of the RhoA pathway and the activation of the Rac1 pathway blocked the F-actin cytoskeleton assembly.”
“the down-regulation of RhoA activity and/or pressure significantly blocked the phosphorylation of ERK1/2″<-LSJL increases ERK1/2 phosphorylation.
“RhoA activation inhibited the pressure-induced down-regulation of P-ERK1/2 expression and that RhoA played an important role in the regulation of ERK1/2 phosphorylation upon pressure stimulation.”
“A combination of decreased RhoA activity and pressure stimulation (P/RhoA − group) achieved the maximum expression of the chondrogenic marker genes in BMSCs{So we have to find a way to decrease RhoA levels in the bone}. After two weeks of chondrogenic induction, the expression levels of the chondrogenic genes in the P/RhoA + group were significantly reduced compared with those of the P group. The up-regulation of RhoA antagonized the promoting effect of pressure on the chondrogenic differentiation of the BMSCs{So how do we downregulate RhoA?}. After 4 weeks of chondrogenic induction, the expression levels of Sox-9, Aggrecan and Col II in the RhoA −, P and P/RhoA − groups were significantly higher than those of the control group ”
” pressure inhibited ERK phosphorylation, suggesting that the induction of cell cycle initiation by pressure may not require a modulation of the cyclin D concentration, which involved the regulation of ERK activity through the LIMK protein.”
“pressure promoted the phosphorylation of JNKs but not ERKs in the BMSCs.”
“the damage to the cytoskeletal structure and the inhibition of ROCK activity induced rounded cell morphologies and promoted the expression of chondrogenic marker genes in the BMSCs“<-Maybe hydrostatic pressure will induce more cytoskeletal damage over time and more chondrogenic differentiation will be induced naturally.
“Hydrostatic pressure regulates cell cycle initiation through both the RhoA/Rock and the Rac1 signaling pathways. At the same time, the mechanical stimulation promoted cytoskeletal assembly in BMSCs through the up-regulation of RhoA/ROCK activities, and activation of the JNK1/2 pathway by down-regulation of Rac1 activity. Hydrostatic pressure could also enhance expression of marker genes for early osteogenic differentiation through the up-regulation of RhoA activation or enhance the expression of chondrogenic marker genes in BMSCs during chondrogenic differentiation via the up-regulation of Rac1 activity ”
According to Flavoprotions: Advances in Research and Application: 2011, egf is a stimulator of Rac1.
There are several skin applicators of EGF but I couldn’t find any oral.
I’m not sure if applying it to the skin near the bone/joint region would work in stimulating Rac1 and I’m not sure if ingesting a product meant for the skin is safe nor if it will stimulate any desired region. But the study suggests Rac1 versus RhoA is a key cellular distinction for osteoblasts versus chondrocytes. And since there is as yet no RhoA inhibitor(but it’s being investigated due to cancer applications), stimulation of Rac1 via EGF is a worthwhile path to go down. By encouraging chondrogenesis versus osteogenesis in the bone, it would be easier to great a neo-growth plate.
Rac1 promotes chondrogenesis by regulating STAT3 signaling pathway. has a possible suggestion of how STAT3 could also promote chondrogenesis,
“The small GTPase protein Rac1 is involved in a wide range of biological processes including cell differentiation. Previously, Rac1 was shown to promote chondrogenesis in micromass cultures of limb mesenchyme. However, the pathways mediating Rac1’s role in chondrogenesis are not fully understood. This study aimed to explore the molecular mechanisms by which Rac1 regulates chondrogenic differentiation. Phosphorylation of signal transducer and activator of transcription 3 (STAT3) was increased as chondrogenesis proceeded in micromass cultures of chick wing bud mesenchyme. Inhibition of Rac1 with NSC23766, janus kinase 2 (JAK2) with AG490, or STAT3 with stattic inhibited chondrogenesis and reduced phosphorylation of STAT3. Conversely, overexpression of constitutively active Rac1 (Rac L61) increased phosphorylation of STAT3. Rac L61 expression resulted in increased expression of interleukin 6 (IL-6), and treatment with IL-6 increased phosphorylation of STAT3. NSC23766, AG490, and stattic prohibited cell aggregation, whereas expression of Rac L61 increased cell aggregation, which was reduced by stattic treatment. Rac1 induces STAT3 activation through expression and action of IL-6. Overexpression of Rac L61 increased expression of bone morphogenic protein 4 (BMP4). BMP4 promoted chondrogenesis, which was inhibited by K02288, an activin receptor-like kinase-2 inhibitor, and increased phosphorylation of p38 MAP kinase. Overexpression of Rac L61 also increased phosphorylation of p38 MAPK, which was reduced by K02288. These results suggest that Rac1 activates STAT3 by expression of IL-6, which in turn increases expression and activity of BMP4, leading to the promotion of chondrogenesis.”
So BMP4, STAT3, and IL6 are all potential targets to induce chondrogenesis.
Contribution of the Interleukin-6/STAT-3 Signaling Pathway to Chondrogenic Differentiation of Human Mesenchymal Stem Cells.
“Mesenchymal stem cells (MSCs) are multipotent cells that can differentiate into chondrocytes. Articular cartilage contains MSC-like chondroprogenitor cells, which suggests their involvement in the maintenance of cartilage homeostasis by a self-repair mechanism. Interleukin-6 (IL-6) is a cytokine [which is] produced by MSCs in a steady manner and in large quantities. The purpose of this study was to investigate the involvement of IL-6 signaling in MSC differentiation into chondrocytes.
Human bone marrow-derived MSCs were cultured using a pellet culture system in medium containing transforming growth factor β3. Chondrogenic differentiation was detected by cartilage matrix accumulation and chondrogenic marker gene expression.
IL-6 was detected at a high concentration in culture supernatants during chondrogenic differentiation. The expression of the IL-6 receptor (IL-6R) was significantly increased, accompanied by markedly increased phosphorylation and expression of STAT-3. Addition of IL-6 and soluble IL-6R (sIL-6R) to the chondrogenic culture resulted in concentration-dependent increases in cartilage matrix accumulation and cartilage marker gene expression (type II collagen/aggrecan/type X collagen). Phosphorylation of the master transcription factor SOX9 was enhanced upon addition of IL-6 and sIL-6R. STAT-3 knockdown suppressed chondrogenic differentiation. IL-6 and the MSC markers CD166 and nestin were colocalized in macroscopically normal human cartilage taken from the lateral femoral compartment of knees with medial tibiofemoral osteoarthritis.
During differentiation of human MSCs into chondrocytes, the activation of IL-6/STAT-3 signaling positively regulated chondrogenic differentiation. The presence of IL-6 around MSC-like cells in the cartilage tissue was identified, suggesting that IL-6 contributes to homeostasis and cartilage self-repair by promoting chondrogenic differentiation.”
“cartilage contains chondrogenic progenitor cells with mesenchymal stem cell (MSC)–like characteristics”<-possibly actually more like epithelial cell characteristics.
“MSCs exhibit immunosuppressive activity and inhibitory effects on osteoclast differentiation via trophic effects, by releasing various humoral factors”
“MSCs produced high levels of IL-6 during chondrogenic differentiation”
“Chondrocytes are also capable of producing IL-6 upon stimulation, although under physiologic conditions (i.e., embedded in the cartilage matrix), chondrocytes are reported to have little ability to produce IL-6”
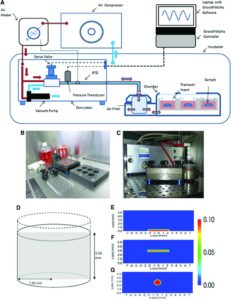
Evaluation of the Growth Environment of a Hydrostatic Force Bioreactor for Preconditioning of Tissue-Engineered Constructs
“To determine the effect of hydrostatic pressure on bone formation, chick femur skeletal cell-seeded hydrogels were subjected to cyclic hydrostatic pressure at 0-270 kPa and 1 Hz for 1 h daily (5 days per week) over a period of 14 days. At the start of mechanical stimulation, dissolved O2 and CO2 in the medium increased and the pH of the medium decreased, but remained within human physiological ranges.”
“Hydrostatic pressure has been shown to be an important mechanical stimulus for the direction of cell fate in various tissues, including articular cartilage, the intervertebral disc, bone, and the vascular system”<-We want to induce the cell fate of chondrogenesis.
“Osteocytes in the canalicula-lacuna network of load-bearing bones are subjected to physiological pressures of approximately 270 kPa”
“he heartbeat of chick embryos delivers a dynamic pressure of 4 kPa, the blood pressure is usually between 8–24 kPa, the hydrostatic pressure in the cerebrospinal fluid is around 1.2 kPa and the interstital fluid pressure is around 0.27 kPa”
“The application of hydrostatic pressure during tissue formation could result in enhanced transfer of small molecules, such as oxygen and CO2, into the tissue matrix and provide physical forces to cells and tissues”
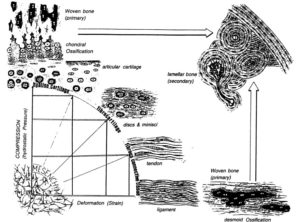
Biomechanics-driven chondrogenesis: from embryo to adult.
“Cartilage is relatively acellular, with chondrocytes only comprising 1–5% of the tissue by volume”
“Joint loading results in direct compression of chondrocytes inside a relatively impermeable matrix. Following tissue loading, hydrostatic pressure initially develops in the interstitial fluid, which is followed by fluid flow-induced shear. However, in time scales > 10 μs, the solid matrix begins to bear the applied load, resulting in deformation. Consequently, the cells residing in the matrix experience hydrostatic pressure, shear, compression, and, to a lesser extent, tension. This mechanical stimulation produces a signaling cascade, resulting in increased gene expression, matrix protein production, and intracellular ion influx”<-Our goal though is to induce hydrostatic pressure within the bone to induce chondrogeneic differentiation.
“Spatiotemporal changes in progenitor cell adhesion molecule expression cause similar cells to transiently associate during chondrogenesis. However, cell-cell adhesion strength correlates linearly with cellular surface tension, irrespective of a homogeneous or heterogeneous interaction, suggesting surface tension as the primary driver of differential adhesion. Therefore, precartilaginous condensation may be the result of mesenchymal progenitor cells exhibiting similar surface tensions rather than similar biomarkers. Furthermore, disruption of surface tension inhibits differential adhesion“<-so altering surface tension may be key to inducing chondrogenesis and cellular biomarkers may not necessarily be a limiting factor on chondrogenic induction.
“the absence of gravitational force reduces precartilaginous condensations in mesenchymal limb bud cells”
“Biomechanics-driven development of cartilage from embryo to fetal stages and beyond. A, B) Progenitor cells migrate from the early mesoderm to sites of skeletogenesis (A), where they undergo precartilaginous condensations (B). C) Chondrocyte progenitors secrete cartilage-specific matrix and decrease expression of cell-cell interaction proteins. D) Proliferation continues at the subchondral growth front, while endochondral ossification occurs throughout the juvenile stages to transform cartilage into bone. E) Ends of long bones remain capped with a layer of articular cartilage throughout adulthood.”
” Intermittent and cyclic hydrostatic pressure and strain both help regulate matrix protein synthesis to affect macromolecular organization of collagen fibers, which, in turn, leads to changes in the mechanical properties of the tissue ”
“models predict that intermittent hydrostatic pressure inhibits degeneration and ossification of cartilage, while intermittent strain or shear stresses accelerate ossification and degeneration”
“HP does not result in deformation of incompressible media, so it is not expected to deform cells. Direct compression results in deformation of matrix and cells, which will also create fluid flow that is not observed with HP.”
Response to mechanical loading may be differentiation dependent.
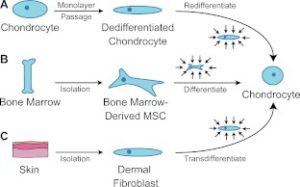
The above image is our intent where we intent to get bone marrow MSC’s to differentiate into chondrocytes. However, we cannot isolate them in culture.
“Harnessing biomechanics to drive adult cell chondrogenesis. A) Following monolayer culture, chondrocytes rapidly dedifferentiate. Biomechanical stimuli, such as HP, can promote redifferentiation. B) Under mechanical stimulation, mesenchymal stem cells migrate and chondrodifferentiate. C) Mechanical stimulation can be used to induce transdifferentiation into chondrocytes.”
Human mesenchymal stem cell responses to hydrostatic pressure and shear stress.
“the present study investigated the early responses of human mesenchymal stem cells (hMSCs) to intermittent shear stress (ISS) and to cyclic hydrostatic pressure (CHP) simulating some aspects of the biological milieu in which these cells exist in vivo. Production of nitric oxide (NO) and mRNA expression of several known mechanosensitive genes as well as ERK1/2 activation in the hMSC response to the two mechanical stimuli tested were monitored and compared. NO production depended on the type of the mechanical stimulus to which the hMSCs were exposed and was significantly higher after exposure to ISS than to CHP. At the conditions of NO peak release (i.e., at 0.7 Pa for ISS and 50,000 Pa for CHP), ISS was more effective than CHP in up-regulating mechanosensitive genes. ERK1/2 was activated by ISS but not by CHP. The present study is the first to report that PGTS2, IER3, EGR1, IGF1, IGFBP1, ITGB1, VEGFA and FGF2 are involved in the response of hMSCs to ISS. These findings establish that, of the two mechanical stimuli tested, ISS is more effective than CHP in triggering expression of genes from hMSCs which are bioactive and pertinent to several cell functions (such as cell differentiation and release of specific growth factors and cytokines) and also to tissue-related processes such as wound healing.”
“Exposure of the cell distal membranes to shear stress was achieved by flowing the cell-culture liquid medium through low-wall microchannels (μ-Slide) on whose surfaces (2.5 cm2) the cells were cultured; during these experiments, the medium flow was in the axial direction of the microchannels (μ-Slide).”
“PTGS2, IER3, EGR1, IGF1, IGFBP1, ITGB1, VEGFA and FGF2 genes are expressed in the response of human MSCs exposed to ISS, and that (ii) the FGF2 gene (but neither PTGES, EGR1 or VEGFA genes) are involved in the response of hMSCs to CHP. Last, but not least, the observed expression of mechano-sensitive genes (specifically, PTGS2, IER3, EGR1, IGF1, IGFBP1, ITGB1, FGF2 and VEGFA) returned to basal levels within 6 to 24 h after exposure of the hMSCs to the two mechanical stimuli tested”





