What I will be posting in this and the next post are devices I have found being sold from different companies which have devices which can accelerate the healing rate of bone fractures. The devices are something that can be attached to the skin of the area of the body the person is trying to stimulate bone growth in.
Analysis & Interpretation
This device seems to be able to use the PEMF (pulsed electromagnetic field) concept to heal bones. This is similar to the idea of using the LIPUS theory for bone fracture healing.
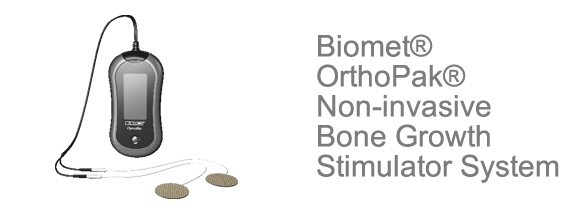
With the Bone Growth Stimulator System (OrthoPak), the device is used to help accelerate the rate of bone non-unions heal. You have two round electrodes you attach to the skin about 2-3 inches apart from each other and then turn on the device to cause a current to go under the skin connecting the electrodes into a closed circuit (I am guessing). The electrodes are supposed to be disposable and easy to adhere to the skin (with some type of weak glue I am guessing). The electrical signal is supposed to be of high frequency.
With the Spine Fusion Stimulator System (SpinePak) the device is exactly the same but the application of it is different. The main difference seems to be wear you are supposed to attach the electrodes around the lower back region, and maybe also the magnitude of the current the wires are supposed to induce.
Implications For Height Increase
This device which requires a doctor prescription to get is one of the easy to use, lightweight devices which can possible cause a little bit of height increase if the theory makes any sense. Somehow there have been a few sporadi studies and articles published even from 80 years ago which suggest that inducing a low magnitude electrical signal. An old post about the possibility of using the PEMF theory as a way to increase height was written “Increase Height And Grow Taller Using Pulsed Electromagnetic Field Therapy, PEMF“. To iterate the message I had written in that post and add some more content to that old message, maybe the application of a slightly stronger electrical field at a more dynamic but consisten usage to the interverterbal disk area can cause the annulus to increase in width and lead to some temporary increase height of a few millimeters.
From the company Biomet website HERE…
For the Biomet Orthopak Non-Invasive Bone Growth Stimulator System FAQ Broshure
The Biomet® OrthoPak®System promotes normal healing by inducing weak pulsing electrical signals at the nonunion fracture site. These signals are generated by a low energy electromagnetic field created by passing specific electrical current pulses through a flexible therapeutic treatment coil. A specific electrical current is delivered to the coil by the control unit. The coil then delivers the therapeutic electromagnetic signal to the nonunion fracture site.
Easy To Use and Operate
The Biomet® OrthoPak® System is portable, easy to use and operate; thus allowing you to treat your fracture nonunion while going about your daily routine. You can use the system with a cast or with a brace. The Biomet® OrthoPak® System capacitive coupling technology is the only non-invasive technology that promotes normal healing at the nonunion site 24 hours per day as recommended. The Biomet® OrthoPak® System can only be obtained with a doctor’s prescription. Upon receiving your Biomet® OrthoPak® System you will receive a Patient’s Guide containing complete instructions. Your compliance with these instructions will contribute to the successful outcome of your treatment.
System Advantages
- The Biomet® OrthoPak® System is especially appropriate for treating proximal femur (center of the thigh bone), metatarsal/tarsal (arch of the foot or ankle) and clavicle (collarbone) fractures
- The Biomet® OrthoPak® System is the smallest and lightest, low-profile non-invasive bone growth stimulator on the market today
- The Biomet® OrthoPak® System electrode placement is easy and universal to all application areas – one versatile system can treat various fracture nonunions
- The Biomet® OrthoPak® System is supplied with a unique ergonomic, rotating holster designed for active lifestyles
- Patient usage and therapeutic treatment time may be downloaded to a PC for viewing, storage or printout via use of the Biomet compliance Data Download Software and USB Cable
- Two different types of disposable electrodes
The Bone Healing Process
Bone is one of the tissues within the human body capable of healing itself when damaged, in much the same way as skin and other tissues. When you fracture a bone, the body naturally produces low level electric impulses that help to heal the fracture site. Occasionally, the fracture may not heal on its own. This will result in what is called a nonunion. The Biomet® OrthoPak® Non-invasive Bone Growth Stimulator System is a proven, safe, and effective method for promoting the healing of fractures that have progressed to a nonunion.
How does the Biomet® OrthoPak® Non-invasive Bone Growth Stimulator System Work?
The Biomet® OrthoPak® Non-invasive Bone Growth Stimulator System delivers an electrical treatment signal that is intended to help your fracture nonunion heal. Two lightweight electrodes, which look like round bandages are dermally applied and placed 2-3 inches apart and over from the fracture nonunion site. The electrodes are easy to apply and are extremely lightweight. The Biomet® OrthoPak® Non-invasive Bone Growth Stimulator System is battery operated with a rechargeable battery. Upon insertion of a charged battery, the Biomet® OrthoPak® Non-invasive Bone Growth Stimulator System is automatically activated and ready to deliver therapeutic treatment. The Biomet® OrthoPak® Non-invasive Bone Growth Stimulator System is portable and easy to operate. You will be able to treat your fracture nonunion while going about your daily routine, relaxing or while sleeping.
Biomet® SpinalPak® Non-invasive Spine Fusion Stimulator System
Click for the Biomet SpinalPak Non-invasive Spine Fusion Stimulator System FAQ Broshure
By prescribing the Biomet® SpinalPak® Non-invasive Spine Fusion Stimulator System, your doctor has taken advantage of an adjunctive non invasive treatment to aid in the healing of your primary lumbar spine fusion surgery. The Biomet® SpinalPak®System delivers a low intensity electrical signal that promotes normal healing at the fusion site. The therapeutic signal operates at a high frequency; therefore, you should not feel anything during your treatment. Two small, lightweight disposable electrodes (conductors of the electrical signal) are placed on your lower back, inches apart from one another, at the level of your surgery. These electrodes are easy to apply and adhere to the skin.
Easy To Use and Operate
The Biomet® SpinalPak® System is portable, easy to use and operate; thus, allowing to help you heal while going about your daily routine. The system’s lightweight, low-profile, disposable electrodes allow treatment to occur in conjunction with wearing a back brace or just stand alone. The Biomet® SpinalPak® System’s capacitive coupling technology is the only non-invasive technology that promotes normal healing at the fusion site 24 hours per day as recommended. The Biomet® SpinalPak® System can only be obtained with a doctor’s prescription. Upon receiving your Biomet® SpinalPak® System you will receive a Patient’s Guide containing complete instructions. Your compliance with these instructions will contribute to the successful outcome of your treatment.
System Advantages
- The Biomet® SpinalPak® System is the smallest and lightest, low-profile non-invasive spine fusion stimulator on the market today
- The Biomet® SpinalPak® System is supplied with a unique ergonomic, rotating holster designed for active lifestyles
- Patient usage and therapeutic treatment time may be downloaded to a PC for viewing, storage or printout via use of the Biomet Compliance Data Download Software and USB Cable
- Two different types of disposable electrodes
How does the Biomet® SpinalPak® Non-invasive Spine Fusion Stimulator System work?
Spinal fusion joins one or more lumbar vertebrae to eliminate motion, increase stability and to try to reduce pain. Following spinal fusion surgery, in the lower (lumbar) spine, the Biomet® SpinalPak® Non-invasive Spine Fusion Stimulator System may be prescribed to assist in healing the fusion by sending electrical impulses directly to the spine that mimics your body’s natural healing process. Two lightweight dermally applied electrodes similar in size to a quarter are placed on your back adjacent to the surgical site. The electrodes are easy to apply and are extremely lightweight. The Biomet® SpinalPak® Non-invasive Spine Fusion Stimulator System is battery operated with a rechargeable battery pack. Upon connection of the charged battery pack, the Biomet® SpinalPak® Non-invasive Spine Fusion Stimulator System is automatically activated and ready to deliver therapeutic treatment.
How long will I have to wear the device?
The Biomet® SpinalPak® Non-invasive Spine Fusion Stimulator System is designed to deliver 270 days of continuous therapeutic treatment. The indicated wear time is 24 hours per day. Certain factors such as smoking, instrumentation and multiple level fusions may factor into the duration of your treatment. Biomet recommends that your doctor closely monitor your healing progress to determine how long you should treat with the device. It is important that you use the device as prescribed.




