Indirubin has shown promise before as a CXXC5 inhibitor. ” elongated tibial length through delayed senescence and further activation of the growth plate in adolescent mice.”<-further activation of the growth plate is what is most interesting.
We have to be on the lookout for indirubin derivative, KY19382.
Here’s a patent PHARMACEUTICAL COMPOSITION CONTAINING INDIRUBIN DERIVATIVE AS ACTIVE INGREDIENT
Interesting parts of the patent:
“The composition of claim 7, wherein the compound promotes bone growth and/or increases bone thickness.
12. The composition of claim 7, wherein the compound promotes chondrocyte proliferation, induces differentiation of osteoblasts, and/or activates β-catenin stabilization and nuclear translocation of β-catenin.”
“The composition for promoting bone length growth in accordance with the present invention has the promoting effects on both proliferation of chondrocytes and differentiation of osteoblasts, thereby increasing both bone length and thickness (bone density). Therefore, because it has the advantages of a wide range of prescriptions for various age groups, it can be usefully used as a pharmaceutical composition that can promote the growth of bone length and thickness.”
“The food compositions of invention, if manufactured as a beverage, is essential at the rate indicated, and there is no specific restriction on the liquid composition, such as ordinary drinks, and may contain several flavoring agents or natural carbohydrates as an additional ingredient. Examples of natural carbohydrates described above are monosaccharides (e.g. glucose, fructose and the like), disaccharides (e.g. maltose, sucrose and the like), and polysaccharides (e.g. dextrin and cyclodextrin, as well as common sugars such as xylitol, sorbitol and erythritol). As flavoring agents other than those described above, natural flavoring agents (e.g. taumatine, stevia extracts, such as fume-based A and glycirrhizine, and synthetic flavoring agents, such as sakarin, aspartame) can be advantageously used. The ratio of the above natural carbohydrates is generally about 1 to 20 g per 100 ml of the composition of this invention and, preferably, about 5 to 12 g.”
So seems like it will be a beverage which could mean it’s for everyone!!!!!!!!!!
Here’s another interesting study:
Indirubin-3′-oxime stimulates chondrocyte maturation and longitudinal bone growth via activation of the Wnt/β-catenin pathway
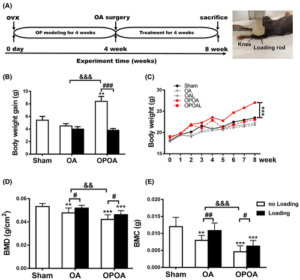
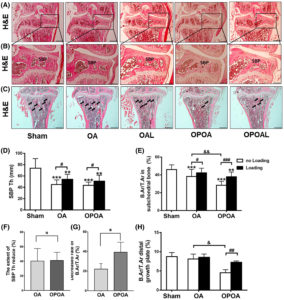
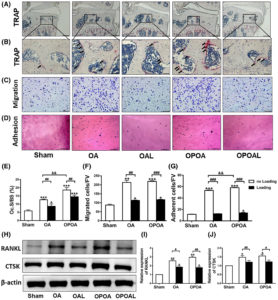
“we identified indirubin-3′-oxime (I3O) as a compound capable of enhancing longitudinal bone growth. I3O promoted chondrocyte proliferation and differentiation via activation of the Wnt/β-catenin pathway in vitro. Intraperitoneal injection of I3O in adolescent mice increased growth plate height along with incremental chondrocyte maturation. I3O promoted tibial growth without significant adverse effects on bone thickness and articular cartilage. Therefore, I3O could be a potential therapeutic agent for increasing height in children with growth retardation.”
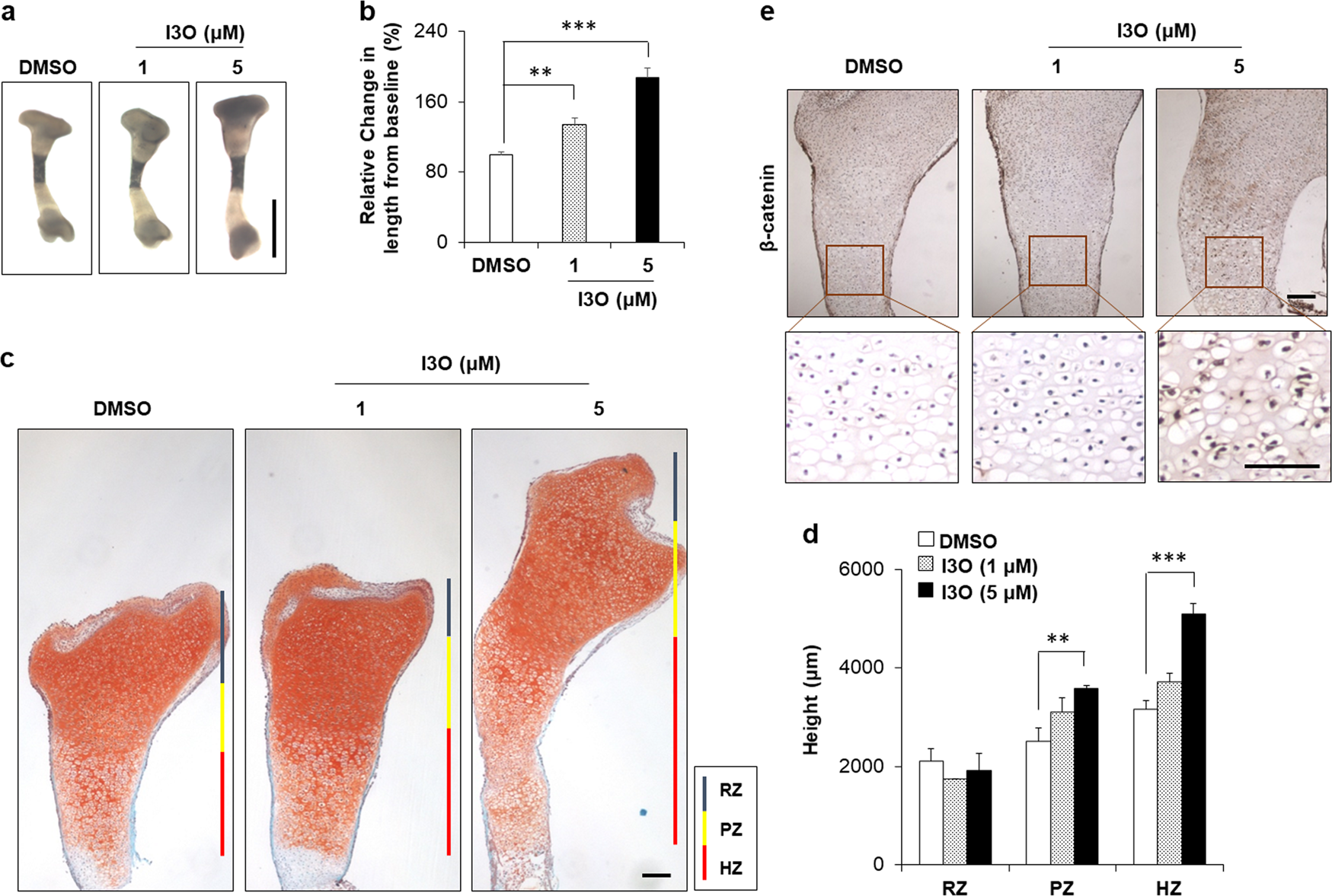

Look at the tibia growth in fig2 at 5uM it is sick! Although a bit curved. If you look at 2a it looks fairly normal though.
“I3O significantly increased the mRNA levels of early chondrogenesis markers, such as Col2a1 and Sox9. In addition, I3O treatment increased the mRNA levels of fully differentiated hypertrophic chondrocyte markers, such as Col10a1, Vegfa, Alp, and Mmp13, as well as prehypertrophic chondrocyte markers, such as Runx2 and Mmp9. Transcriptional induction of these genes by I3O treatment was suppressed by knockdown of Ctnnb1″
“The practical usage of I3O is advantageous because it is an indirubin derivative that is the main active ingredient in a traditional Chinese medicine, Danggui Longhui Wan”<-even if indirubin is in the Danggui Longhui Wan we still don’t know if it’s effective.
All height seekers should be monitoring Indirubin
Here’s a biohacker thread on indirubin. One interesting comment: “The issue seems to be that the drug has not been studied in humans, nor in vivo for this application. With the current understanding of hormono-dynamics, GH secretagogues + antidiabetic compounds (metformin) seem to be the best bet to enhance height safely in adolescents.” On metformin “Metformin in this case would mediate any deleterious effects on glucose metabolism exerted by the gh secretagogues which are known to mess up glucose tolerance.”
Here’s some studies with updates on Indirubin:
ERα/β/DMP1 axis promotes trans-differentiation of chondrocytes to bone cells through GSK-3β/β-catenin pathway
“Estrogen-induced premature closing of the growth plate in the long bones is a major cause of short stature after premature puberty. Recent studies have found that chondrocytes can directly trans-differentiate into osteoblasts in the process of endochondral bone formation, which indicates that cartilage formation and osteogenesis may be a continuous biological process. However, whether estrogen promotes the direct trans-differentiation of chondrocytes into osteoblasts remains largely unknown. Chondrocytes were treated with different concentrations of 17β-estradiol, and Alizarin Red staining and alkaline phosphatase activity assay were used to detected osteogenesis. Specific short hairpin RNA and tamoxifen were used to block the estrogen receptor (ER) pathway and osteogenic marker genes and downstream gene expression were detected using real-time quantitative polymerase chain reaction, western blot, and immunohistochemistry staining. The findings showed that 17β-estradiol promoted the chondrocyte osteogenesis in vitro, even at high concentrations. In addition, blocking of the ERα/β pathway inhibited the trans-differentiation of chondrocytes into osteogenic cells. Furthermore, we found that dentin matrix protein 1 (DMP1), which is a direct downstream molecular of ER, was involved in 17β-estradiol/ER pathway-regulated osteogenesis. As well, glycogen synthase kinase-3 beta (GSK-3β)/β-catenin signal pathway also participates in ERα/β/DMP1-regulated chondrocyte osteogenesis. The GSK-3β/β-catenin signal pathway was involved in ERα/β/DMP1-regulated chondrocyte osteogenesis. These findings suggest that ER/DMP1/GSK-3β/β-catenin plays a vital role in estrogen regulation of chondrocyte osteogenesis and provide a therapeutic target for short stature caused by epiphyseal fusion.”
<-couldn’t get this full study but it had stuff on Indirubin.
Indirubin-3′-alkoxime derivatives for upregulation of Wnt signaling through dual inhibition of GSK-3β and the CXXC5-Dvl interaction
“Glycogen synthase kinase-3β (GSK-3β) appears to be ordinarily expressed, and functionally redundant in Wnt/β-catenin signaling. The Wnt proteins induce transduction of a cytoplasmic protein, Dishevelled (Dvl) which negatively modulates GSK-3β activity. CXXC5 is a negative modulator of the Wnt/β-catenin signaling through the interaction with Dvl in the cytosol. This indicates that Wnt/β-catenin signaling could be efficiently modulated by controlling GSK-3β and the CXXC5-Dvl interaction. In this study, we designed a series of indirubin-3′-oxime and indirubin-3′-alkoxime derivatives containing various functional groups at the 5- or 6-position (R1) alongside alkyl or benzylic moieties at the 3′-oxime position (R2). These activate Wnt signaling through inhibitions of both GSK-3β and the CXXC5-Dvl protein–protein interaction, in addition, the improvement of pharmacological properties. The potent activity profiles of the synthesized compounds suggested that dual inhibition of GSK-3β and the CXXC5-Dvl interaction could be an appropriate approach towards safely and efficiently activating Wnt signaling. Thus, dual-targeting inhibitors are potentially better candidates for efficient activation of Wnt signaling compared to GSK-3β inhibitors.”
Also couldn’t get this full study