Personal Message: I wanted to write this post as a final message to my own doubts of the effectiveness and feasibility of the LSJL method. After this, I will move away from talking about the method too much because I don’t feel I am qualified or knowledgeable enough on the subject to really study it in depth.
From this previous post HERE, Tyler and I had an exchange of emails back and forth with me trying to fully understand why the Lateral Synovial Joint Loading technique would or could ever even work. The main concerns I had were never put to rest and I wanted to make a clear statement for the readers now that there is quite a bit of data showing that by theory alone, the method should not work, at least at the level of understanding on how bone mechanics work at this point.
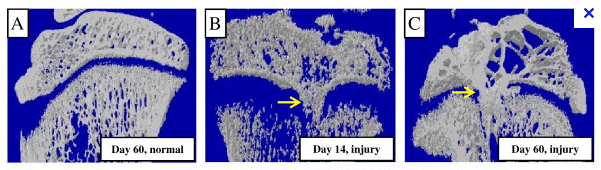 The reason is because of a type of effect from growth fractures known as bone bridges. I wanted to show the studies that have been done which showed growth plate fractures which caused what are known as ” bony bridge” which is a sort of like a real bridge that connects the bone epiphyseal end with the bone metaphyseal ends of the long bone together and they lead to almost completely stunted linear growth from just that small piece of bone connection. Before that they were technically separated by cartilage. It is also important to remember that even though the two bones are connected with bone, there is still an entire rest of growth plate surrounding the bone bridge, which doesn’t work well anymore,
The reason is because of a type of effect from growth fractures known as bone bridges. I wanted to show the studies that have been done which showed growth plate fractures which caused what are known as ” bony bridge” which is a sort of like a real bridge that connects the bone epiphyseal end with the bone metaphyseal ends of the long bone together and they lead to almost completely stunted linear growth from just that small piece of bone connection. Before that they were technically separated by cartilage. It is also important to remember that even though the two bones are connected with bone, there is still an entire rest of growth plate surrounding the bone bridge, which doesn’t work well anymore,
The bony bridge effectively completely stopped the long bone from ever being able to lengthen, at least in that specific area where the epiphysis and metaphysis is attached.
What I am trying to say is that the LSJL method is effectively trying to push against bone that has completly surrounded it, in all 3 dimensions. Just from a broad general analysis point of view, I was arguing in the previous post with Tyler that the reasons growth plates work is that they completely seperate two pieces of bone. From a mathematically topological viewpoint I could say that the bones are solid in the radial direction but in the axial direction, they are completely separated by the growth plate. surrounding the 3 parts radially is the muscles and ligaments that go around it, but they are elastic and can stretch.
If we now look at how the method of LSJL is supposed work (from my understanding after having the discussion with Tyler in the pervious post), chondrocytes proliferated and expanded in the epiphysis internal region of the long bone should be able to push the thick bone sorrounding it in the epiphysis in all directions 3 dimensionally out thus expanding out the entire bone, but the LSJL method is hoping the epiphyseal bone will be the main parts that are suppose to be expanded. This means that the overall bone structure surroundin the chondrocyte and cartilage that is supposed to have developed has fundamentally changed, from not just 2 dimensionally anymore but 3 dimensionally Where there was once muscle which was elastic allowing for longitudinal stretching thus growth there is not bone, which is not as aleastic as muscle. That was my original concern.
For studies showing evidence that even a slight bone bridge between the two bone ends separated by the cartilage starts to get connected by the bone, growth is almost completely stunted. Topologically (and using some physics lingo) speaking the one direction the chondrocytes can expand in has been fixed with a constraint. If the originally perfectly aligned system of chondrocytes can’t even push past and beyond a bony bridge that has formed on just one side of the natural gorwth plates, what hope and effect does producing chondorcytes inside the epiphysis using LSJL can possibly have then where the bones are completely surrounding it?
As for the claim that Tyler has grown 1.5 inches from the Lateral Synovial Joint Loading, LSJL technique I really can’t explain that away. I could make the argument that there are plenty of stories of people who have gone through a slight or mini growth spurt really late in their 20s or even in their 30s. There are obviously some documented cases in the medical literature. One guy on a bodybuilding/ steroids internet forum/board talked about his mother who grew a little (1-2 inches) when she was in her mid to late 20s (can’t find the link at this time). If someone asked me to explain his (tyler’s) claim, another logical explanation to explain away the claim is to say that he is a liar and lying to you, but I would assume that Tyler is a honest person who would not lie about this type of thing, especially since he has been so dedicated for so long in finding a solution using real science and theory. I will just say that he is not lying to you. He is telling the truth about his height increase claim.
This reminds me of my claim behind the story of the Grow Taller Guru Lance Ward. He claims to be able to help you grow up to 6 inches within 90 days. I don’t know about that but in my review on him, I just guessed that when he was in his 20s, say 20 or 21, he felt dissatisfied with his height, he decided to do some streching and excercised and happend to go through an amazing growth spurt of 6 inches in a very short amount of time. If that really happened to him, then I would just curtly proclaim that he was just really lucky. He happened to be one of those people who wants to be taller, tried something out, was not supposed to grow anymore, but did grow and not just a little bit, but a lot in a really short time frame. If that is NOT the case, then I would say Lance lied about his story and did not increase in the 6 inches he claimed since he is really marketing his services in traditional internet marketing fashion which makes me distrust him.
I woudl guess that there are probably millions of below average height adolescent and teeenagers who secretly desire to be taller and try all sorts of exercises and stretching to increase in height. For some of them who are lucky and still have some growth left, they might be able to incerase by quite a bit of height. Michael Jordan desperately wanted to be taller (actually 7 foot) when he was a sophomore in high school being only 5′ 11″. He would hang on a bar in the house backyard and he grew 5 inches in 1 year but mostly in the summer. Dennis Rodman was said to have grown 10 inches in around 1 years time from the age of 20-22. Without that height he deinitely wouldn’t have been ever a NBA player .The 2012 NBA #1 draft pick Anthony Davis was a nobody in early high school being a 6’2 or 6′ 3″ point guard but his enormous and extremely lucky growth spurt of 7-8 inches shot him up to the best of the basketball world. Without the growth spurt, David might not have even been able to play college basketball. What I am trying to say is that maybe, just maybe that the growth that Tyler that has gotten is from just luck. There is always the arguement that life happens in strange ways. Sometimes strange things happen which look like miracles.
Now to play the devil’s advocate position against my previous argument…
Obviously the strange thing about his growth at such a later life is that unlike other people who might be in their late 20 s who have wanted to grown taller and did grow taller, he just happened to be one of the only people in the world who writes a Height Increase and Grow Taller blog/website and uses real science to find a solution. Not only that, he found a science and experiment backed possible height increase method and has been using it for years. If I was a betting man, I would say that the coincedence is too high. He has gotten something right, always assuming he has not been lying about his increase the entire time.
What are the chances that a guy who wants to be taller, does exercises to be taller and really tries, was in his middle to late 20s when he started doing the exercies, who also writes one of the only blogs or websites on the entire internet looking for a height increase solution, is knowledgeable on the real science of human growth, who also has found, claimed, and documented that he has growth in height which is beyond the range of measurement error, and if he is also being completely honest nad not lying about his claim, did actually grow???
How is it possible to just say that all of this was just by pure chance, only randomness. Randomness is the 25 year old women who is happy with her height, never searchs for any height increase information, barely exercises much, and find that she has grown 1-2 inches in her mid to late 20s. That is randomness.
What is supposed to happen with him is too improbable to be just chance or random luck (again, obviously assuming that he is not lying about his increase). He definitely has something which I can’t explain away. I want to prove that the technique/ method doesn’t work but I can’t and I don’t want to disprove it if I can help it. The method gives real hope to people out there who are already physically mature but want to increase their height without the incredible complications that is involved with limb lengthening surgery.
From source link HERE…
Premature Partial Closure and Other Deformities of the Growth Plate: MR Imaging and Three-dimensional Modeling
- Joseph G. Craig, MB, ChB1, Kathryn E. Cramer, MD2, Dianna D. Cody, PhD1, David O. Hearshen, PhD1, Ruth Y. Ceulemans, MD1, Marnix T. van Holsbeeck, MD1 and William R. Eyler, MD1
- Departments of Radiology (J.G.C., D.D.C., D.O.H., R.Y.C., M.T.v.H., W.R.E.)
- Orthopaedic Surgery (K.E.C.), Henry Ford Hospital, 2799 W Grand Blvd, Detroit, MI 48202.
Abstract
PURPOSE: To examine growth plates of the distal femur and tibia with magnetic resonance (MR) imaging to detect bone bridges and other deformities in children.
MATERIALS AND METHODS: Thirteen children (nine boys and four girls, aged 5–13 years; mean age, 9.8 years) were referred because of suspected or known bone bridging of the growth plate. Among the 13 patients, 10 had Salter-Harris fractures of the knee or ankle, two had Blount disease, and one had neonatal sepsis. Fat-saturated spoiled gradient-recalled images enabled reconstruction of a three-dimensional model of the growth plate. Patients underwent one to four MR examinations.
RESULTS: Nine patients had bone bridging of less than 1% to 39% of the area of the growth plate. On MR images obtained in the growth plate of five patients, a stripe of low signal intensity indicated fracture. On MR images obtained in three patients, intrusions of growth plate cartilage into the metaphysis were seen to increase in depth over time. MR images obtained in four patients showed no bridges. In the two patients who underwent surgery, excellent correspondence was found between MR findings and surgical observations.
CONCLUSION: Marked undulation or splitting of the growth plate may occur with fixation of some cartilage in the metaphysis or epiphysis while growth continues. The configuration of the growth plate and bone bridges can be accurately mapped with MR imaging. Treatment planning is facilitated.
Growth arrest followed physeal injury at the knee and ankle in 1.4% of the large series of patients studied by Mizuto et al (1). The occurrence of arrest following Salter-Harris fractures at the knee and ankle is related to many factors, including the type of physeal fracture, the age of the patient, the physis involved, and the amount of energy applied to the bone (2,3). The knee is the most common site of growth arrest (4,5), with the ankle second (4). The development of bone bridges at these sites results in interference with longitudinal growth (4,5).
Physeal fracture is the most common cause of bone bridging across the growth plate, but growth arrest may also be due to other insults, as reported by Ogden (5,6). Such insults include infection, therapeutic irradiation, metabolic or hematologic abnormality, tumor, burn, frostbite, electrical injury, sensory neuropathy, microvascular ischemia, or insertion of metal. Pease (7) reports premature fusion of the growth plate in patients with hypervitaminosis A. Caffey (8) describes cupping of metaphyses following trauma, osteomyelitis, poliomyelitis, vitamin A toxicity, sickle cell anemia, achondroplasia, or osteopetrosis.
If the growth plate is affected eccentrically, tethering will cause angular deformity. If the growth plate is affected centrally, growth at the periphery causes cupping of the metaphysis with shortening of the bone (5). The younger the child, the more severe the complications.
Plain radiography remains the initial imaging approach. Interpretation problems arise if part of the physis is not parallel to the x-ray beam. The presence of growth arrest lines is helpful; if a growth arrest line extends across the entire metaphysis and is parallel to the physis, physeal bridge formation is unlikely (9). As needed, results can be compared with the normal appearance of the growth plate (10,11).
If surgical excision of a physeal bridge is considered, accurate knowledge of its size and position is necessary. Conventional tomography with grid mapping (12), bone scintigraphy (13), and computed tomography with reformatting (14–16) have been used for this purpose. MR has been used to image the growth plate (3,17) and has now become the imaging method of choice (18).
We present our findings in children at high risk for bone bridging in whom we obtained one or more MR studies and 3D models of the growth plate to determine the need for intervention.
From the Ortho Facts Website HERE…
Long-Term Outcome
Growth plate fractures must be watched carefully to ensure proper long-term results.
In some cases, a bony bridge will form across the fracture line that prevents the bone from getting longer or causes the bone to curve. Orthopaedic surgeons have developed techniques to remove the bony bar and insert fat, cartilage, or other materials to prevent it from reforming.
In other cases, the fracture actually stimulates growth so that the injured bone is longer than the uninjured bone. Surgical techniques can help achieve a more even length.
Regular follow-up visits to the doctor should continue for at least a year after the fracture. Complicated fractures (types III, IV, and V) as well as fractures to the thighbone (femur) and shinbone (tibia) may need to be followed until the child reaches skeletal maturity.
From the website for Wheeless Textbook For Orthopaedics on the subject of Physeal Bone-Bridge…
Physeal Bone-Bridge
– bone bridge obliterates growth-plate cartilage & prevents growth;
– peripheral bone bridges predispose patient to angular deformities;
– most common sites of growth arrest include the distal tibia, distal femoral and distal ulnar physis;
– much less common sites include distal radius and proximal humerus;– Radiology:
– extent of bone bridge is demonstrated by CT scanning and tomograms;
– resection is indicated if less than 1/3 to 1/2 of growth plate is involved;
– younger children tend to have a better prognosis w/ resection than older children;
– less than 2 years of remaining growth is a relative contra-indication for bone bridge resection;
– central bars are more amenable to resection than peripheral bars;
– ischemic or septic related bone bars have a poor prognosis w/ resection;– Technical Pearls:
– interposition of fat is easiest and most commonly used agent to prevent bone bridge formation (alternatives include silastic, methyl methacrylate, or free epiphysis)







“”Autogenesis still exists http://autogenesisinfo.com/automator.htmlThough you cant tell from that 90s style web page if they are still in the market. I’d bet they are still the patent holder though. I wrote the software in the original Autogenesis devices. (And have no connection whatsoever to the product or current owners.)””
mclemens1969
26th April, 2012 @ 11:10 am PDT