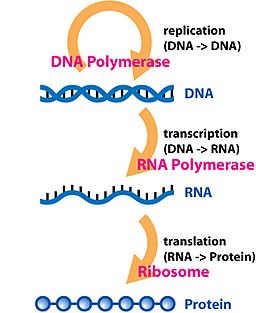
Just for those who have interest in my method
September 8 2006 at 12:26 AM |
Zixialin |
|
Welcome to Network54
We found 12,367 matches for your search height increase qigong on our site.
See What We Found »
|
|
I have put quite a bit of articles on my blog to explain what happened to me. Please read before you have any comment on me.I have interest to guide those who want to spend some sleeping time to grow taller. Since some people complaining me charging too much, now I make it clear: $18 for one year personal letter response, as long as you want to write, as many questions as you want to ask. Besides, I prefer you to give me a report once every 2-4 weeks about what you feel.
I will keep on digging out all details on what happened to me on my blog to give you more and more clear picture about me and to make you believe you can grow taller by your own effort.
Also, let me know what you want me to write about and I will be happy to do so.
My website: http://zixialinmm.blogspot.com
| This message has been edited by zixialinmm on Sep 8, 2006 12:29 AM |
|
|
| Author |
Reply |
| Craig Jordan |
Hmmmm…
|
September 8 2006, 1:24 AM |
|
I’m curious as too see joey’s response on this one……
|
|
| Anonymous |
To Zixialinmm
|
September 9 2006, 11:02 AM |
|
Hey I have checked your website and found it quite interesting, You have mentioned things that Im actually practicing like using The power withn un an absolute power to increase the height of my body. I alway feel like my legs are moving forward when I practic hypnosis as If I am growing because I am growing. I would really like to get to know your method a little more what’s your email? By the way I am a female 19 5’4 and wishing to achive my dream height 5’9 . THank you
|
|
| ed81 |
Qi gong
|
September 9 2006, 12:25 PM |
|
I practise Qi Gong,standing meditation,”Zhang Zhuang,gives you a strong feeling of awareness of your body and the way your Qi (universal energy) flows,(Chinese medical concept)i must admit these exercise give me a very good relaxed feeling,but any HI,allthough having practised it for some years,no way,but it is possible to achieve HI with other Qigong exercises,
who knows,i won’t exclude it.
|
|
| Zixia |
Re: To Zixialinmm
|
September 9 2006, 6:38 PM |
My email is zixialinmm@gmail.com Website: http://zixialinmm.blogspot.com/And please read all of the messages carefully on my blog before you practice, I will put more on in the next few days.
I believe the wish to grow taller is a threshold for us to understand ourself in a much deeper level. And as long as we do keeping on practicing it, we will achieve our goal. That includes you.
Best wishes!
Zixia
|
|
| SD2002 |
Ban Zixialin
|
September 9 2006, 11:47 PM |
|
I think anyone who charges us for anything related to height increased should be banned. I’m tired of Rahul and these others asking us to “pay them” for **** that doesn’t need to be charged for all we know could be bogus. Stop taking advantage of us poor souls.
|
|
| Joey |
Hmmm….
|
September 10 2006, 12:48 AM |
See Craig, this is kinda what I was talking about….some people are gonna be interested enough to maybe wanna try this, but if we were to delete it now…people would say we weren’t being fair.Years ago, I visited the Far East and I saw a number of amazing things people did with the power of the mind alone….things I could never believe before. Do I go with the idea that it is possible to grow with things such as hypnosis…yeah I do, because some of the credible members say they did. Can I say for a fact that this will work or if this person is legit…I really can’t. Usually, I would just say “outright scam, you can’t charge money without any credibility or results”
Of course, say what you will about Rahul though guys….but of all the people that didn’t grow…they got their money back or he’s still working with them…whatever they prefer. Now, honestly…I’m really not that interested or excited, but if so many people start asking about trying this or something….I might just try it before anyone else does just to tell of my experience, but I’d really have to hear something amazing. Is this just someone having fun or is this person really experimenting….I have no clue as of yet, but it seems a lot of work is going into whatever their doing.
As you guys can tell, I’ve gotten a bit more diplomatic, patient and fair in my “old age”.
I will say this though…..I wouldn’t advise a whole lot of people trying it at once. If just one person is gonna try it….then just wait on them. At this point though, I’m not interested enough to entertain the thought now.
|
|
| koolhead |
THE CHARGE
|
September 10 2006, 3:22 AM |
|
EVERYONE WANTS TO SURVIVE ,CHARGING MONEY IS JUSTIFIED…. UNLESS U R NOT A SCAM…
|
|
| ed81 |
suspicious
|
September 10 2006, 4:27 AM |
|
It is justified but also makes ppl suspicious,understandable on this kind of forum.(IMO).
|
|
| koolhead |
yah
|
September 10 2006, 5:38 AM |
|
i agree…. this forum stands against any form of business…. if anyone wants to share his knowlede, experiences and methods ….as a help… for free… then i guess this is the right place
|
|
| frans |
Questions on scams
|
September 10 2006, 10:34 AM |
|
It looks suspicious all right.Im not saying this is a scam or anything but I want to ask if people actually believe in these products and pay for them?
|
|
| Craig Jordan |
I know what you are all thinking
|
September 10 2006, 3:23 PM |
|
Your thinking there is a great possiblity that this a scam. Chances are you stomach feeling is right. But questions pop up. Maybe you’re thinking “boy this girl is cheeky, she came into a scam free board knowing that they’re extremely hated.” I would think that too. Someone has obviously got to be crazy to think that just simple exchanging of letters for advice you probably already know or could have extracted out of a free height increase journal. Then again, i’ll try to give this lady the benefit of the doubt. Listen…. There are many coaches out there, most notably Roger simmons, Tony Robbins, and other personal trainers. Yes they’ll had sucessful lives in it, and some have been the recipient of harsh criticsm by being branded conartists. People need motivation to get them through things because they do not have will power to adhere to a monotonous routine day in and day out. So maybe she did bring good intention to this forum because she is just brazen enough to know they are hated. To be honest with you, however, she offers a proposition that not even the most gullible people can buy into. She seems to not be responding a lot so that is also highly suspicious. If she cared about prospects for her clientele base she would have been importuning us like crazy, but it could be vice versa in the same scenario, regardless she still exudes the presense of a typical scammer. I don’t even know how to call this one because she could very well be the answer to all our gripes about our height. First off, i don’t care who you are, it is terrible to charge money for an unfounded method. She would still have to conduct trials on people to gain credibility. Second off, the way she talks in her blogspot seemed to be chimerical and way out there, but there have been anecdotes where people have grown off mind power. Its really hard to call, thats why i would be more than happy to give you the admonishment that this very well could be a scammer who is plenty aware of duping capability on the members of this forum. If anything you must press her for answers, until she either gives up and relinquishes this charade of being the HI guru, or she remains adamant in what she says. Please be careful
|
|
| ed81 |
it is a scam!!!!!
|
September 10 2006, 4:01 PM |
|
Daiosm,Taoism,it all are very interesting Eastern life-philosophies,studying them they can be useful in your daily life,Qi Gong is a way to control,harmonize and even increase your qi (flow),it is practised for medical reasons (curing diseases) and also martial artists after years of training are able to perform some amazing things.(controling their Qi).
(for instance the Shaolin munks)
But the way this girl illustrate all this,(read it again carefully),and charging money for it,i am sure it is a scam,if you are succesfull with a certain method why not post it on this forum for free (at least i would)
it is still always the same,ppl who are out for some easy money,i must admit they are very inventive to make their theory plausible,but ladies and gents of this forum please don’t fall for this,that would be a pity,again you will loose some money and will not grow a mm.
|
|
| Zixia |
My explanation here again
|
September 10 2006, 4:46 PM |
Ok, if you guys read what I wrote before, you would have got my method completely and practice it for free. But you call me many bad names…
I will put all of my knowledge on my blog for free for your guys to read. What I charge is to respond to individual letters for a year,and I predict it is a lot.Do you call Dr. Laura De Giorgio a scammer? I bought her tapes a few years ago to see how hypnosis did the work. Her tapes were noisy and what she said only reached the beginning of my method on no.6. And she did not even tell you how to use her tapes, no personal support…
Does not matter what you say, I am real and I will do what I think is right. I do not have all day long sitting in front of my computer to write on my blog, but gradually I will put more and more there for you to read. Everyone can grow taller, just like all people get shorter when they are old. You should practice whatever you know to get yourself taller before that hits you again.
My academic background is solid, so if you have any discussion on Taoism, write to me, I will respond you historically and scripturally.
|
|
| ed81 |
zixia
|
September 10 2006, 6:03 PM |
|
Okay,one question,is it true that standing meditation a Qi gong exercise
called in Chinese “Zhang Zhuang”practised for a long time results in HI?Please explain to me the term “Wu Wei”,well don’t on second thoughts Google will give the answer.
|
|
| Zixia |
Re: zixia
|
September 11 2006, 2:27 AM |
When Lao Zi said “wuwei” (no action), he meant two things: things in this world have their own rules (Tao), there is no need for us to take actions in order to make things right. Politically, rulers also should know society has its own rules, so they should not intervene people’s life.However, however, no action does not mean we do not do anything at all (otherwise, a paralyzed person is the best), it means we have to master the Tao in order to understand nature, people, the society, then we know how to take actions. When the world in their right order, we do not need take actions then. If the world is in chaos, that means we made wrong actions, definitely we have to find out what is wrong and correct it.
The later Taoist religion fully developed many many methods to train a Taoist to understand Tao for their purposes. No action is just an appearance when you see a Taoist, he is diligently practicing the method his master teaches him interiorly. If you say wuwei simply explained as “no action” is the center of Taoism, then we have to ignore the Taoist Canon, where you can find all actions.
You brought a good question, reminding me what I thought when I read Daode Jing the first time. I thought suddenly that I could grow taller. Since everything had its Tao, I can do anything I want in this world, nothing can stop me as long as I found its Tao. Tao can guide me to my goal, myself do not need do anything exteriorly eventually. That Tao is Qi (engergy) with guidance of my mind.
|
|
| ed81 |
thanks for the answer,it was very instructive and about my other question?
|
September 11 2006, 5:56 AM |
|
Thanks for the answer,i allready was interested in Taoism and read something about it but your explanation was indeed was very instructive and now my question about “Zuang Zhuang’,practising this and a relation towards HI?
(if there is a relation).
|
|
| Zixia |
About zhanzhuang
|
September 11 2006, 11:10 AM |
|
Sorry missed your question.
I am not sure what HI is. Height increase?
If you say “standing meditation”, it should be “zhanzhuang”. Zhanzhuang is a way of practice Qigong. It exists in almost all kinds of Qigong practices, such as Hexiang (Crane-fly) Qigong, Xiang (Fragrant) Qigong, Yan Xin Qigong, Zhang Hongbao Qigong, and Falun Gong, etc.
Either standing, sitting, or laying down, practice Qigong (in fact it is another name of meditation) is not fixed on one poise. Multi-way of practices can achieve the best result. But each poise has its own advantages than the others. Standing way is the closest to physical excises, so it relaxes your body and helps the blood circulation. Laying down is for those who practice at night when our body is in the mood to sleep and our mind is in the most quite and alert state. Sitting is in the between. I will mention this point in my explanation of my method soon.
|
|
| Joey |
Zixialin
|
September 11 2006, 2:41 PM |
I will say this much, though at this time noone knows hos genuine you or your results really are, you do seem to have a knowledge of what you’re talking about. I mean, when someone pops up out of nowhere and claims to have a method or results….people always think they are some fly by night scammer that will just post and leave….but if you continue to update your site and answer questions…the more people will at least be interested in what you’re doing. Noone here really knows what is going on behind anyone’s else’s computer….and for all we know, you could be completley honest. It’s just that people shouldn’t/can’t make up their minds this quickly. At this time, there really isn’t enough info about you or what you’re doing to justify people paying….at this point. If you do go ahead with all the updates you say and stick around…people will have a better idea.That’s all I’m saying.
So just stick around, answer questions and let us know more about you, your background and exactly what you’re doing. This will make it easier for people to make up their minds.
This message has been edited by tallnowx on Sep 11, 2006 2:44 PM
|
|
|
| ed81 |
zixia,you just want to make some money,read my thread
|
September 11 2006, 9:48 PM |
|
Tao cannot be understand by rational thinking only by intuition,i read some more about it from Lao Tzu,now i am beginning to think you are trying to link Height Increase to Eastern Philosophy in this case Taoism,it is a clever strategy i must admit,a person,you should know this as well as i do,really devoted to Taoism would not ask for money or acknowledgement.Knowing a lot about it also does mean a thing,there’s is enough information on Internet you can quote from,take my advice Zixia and back off,you just
want to make some money,if you really understood the Tao of money,it would be indifferent to you and you wouldn’t make any attempts to get some,only then it will come to you.(not my wisdom but Lao Tzu’s)
|
|

ealges |
ed81.. keep your paws off Ms. Zixia
|
September 11 2006, 10:26 PM |
|
she seems genuine and extremely knowledgeable.. who knows?Keep an open-mind and encourage her to post more stories & tips on her website, instead of attacking her right off the bat (just like how u drove off guys like Rahul & others).. don’t get me wrong, kindd glad u kicked Rahul to the core since his powder is pretty flawed!
|
|
| Zixia |
Nice talking to you all and bye
|
September 11 2006, 10:46 PM |
|
What is intuition and what is rational? When we think we are thinking rationally is because we see some reason causes something else happening many times, so we then can predict something will happen when we see the same cause happens (Since you are so smart, you should know which of your Western philosopher started the theory). The reason we call something happens by intuition because we do not see the link between the cause and result. When you do not see where Tao comes, you do not see where it goes. Once you see…
It is so laughable that you accused me trying to earn money. Anyway, I have decided not to write on this board anymore. For my own privacy, I won’t post my photos and background on my blog at this early stage, but when I feel time is ripe, I will do so.
I love my blog, it brings a lot of good memories and efforts I have made in my life and I will put as much stuff there as possible for those who have interest to read.
Excuse me you guys if you see me putting my blog link in other boards, I want to share my life with comrades.
|
|
| SD2002 |
Stop bastardizing Taoism
|
September 11 2006, 11:15 PM |
|
To use Taoism in the terms of height increase already shows that she’s a scam. Yes there are many schools of Taoism, but Lao Tsu I’m sure would thing using the “tao” for height increase is laughable. Taoism is more of a philosophy similar (or basically the exact same) as Zen Buddhism. It’s like saying you use buddahism to heal the sick when the true nature of Buddahism has nothing to do with that.I agree with using the mind to grow taller, but let’s not throw in Taoism just to make it sound fancy.
|
|
| ed81 |
C’mon eagles,read your own thread
|
September 11 2006, 11:18 PM |
http://www.network54.com/Forum/177048/message/1158015066/jenn..+zixia+is+your+competitor+now-Why your offense thread towards her then and i am convinced she is a scammer just linking Eastern philosophy to HI.
i agree with just like jennifer,leaving so soon this board,because she knows
we understand what she is up to,instead of really working to achieve some HI
with Sky’s methods which of course demands some physical labour,a lot
of forum-members want to get taller by means of mental power,that the way to attract a lot of scammers,of course one can achieve a lot with mental power but beyond certain limits not manipulate a very complex physical process like growing.
But from now on i won’t chase away scammmers anymore,find out for yourselves
in what clever ways ppl try to earn some money on this board
|
|
| SD2002 |
I mean she already gave up and left
|
September 11 2006, 11:23 PM |
I mean cmon, how ridiculous is that? She can’t even back up her views or defend herself without running away? All that crap she talked about Taoism is nonsense. She’s abusing the philosophy with her own ignorance, claiming she grew taller, and charging people with assistance. Do we think Dr. Laura is a scam? Well she wouldn’t refund my money so yeah I think she is.I think we should continue to put pressure on people and see if they crack. If they do then we can at least know their character. We put pressure on Rahul and he flipped. This person ran away saying she’ll advertise on other boards. We don’t need people like this at all. I’d rather have ProHGH supplement scammers post testimonials than have people like Rahul/Zixlaklsjf come and try to propone methods which could easily not work or make people believe it worked.
Like I said, stop taking advantage of people. We’re here in NEED OF HELP. I think it’s sad that there are professionals or groups out there who charge to “cure” people’s sufferings. If you think about it, it sounds great, then you pay them, but they’re the lucky ones with the big houses and nice cars, while we’re stuck in our ruts asking THEM, with ALL OF THEIR MONEY, for help. I say the best philosophy is to help yourself and when you do, help others for free.
|
|
| ed81 |
very good and intelligent thread SD2002,i totaly agree
|
September 11 2006, 11:23 PM |
|
But try to convince the other forum-members,how ridiculous this all is.
That is the real problem.
|
|
| brother_komodo |
Re: Just for those who have interest in my method
|
September 12 2006, 8:03 AM |
comon guys…..seriously, some of the people here have a lot more mental power than this stupid zixia bitch
if growing taller is as simple as she’s making it, without any use of technology or stretching, or supplements, or anything, just your head.
YOUD THINK SOMEONE A FEW MILLION YEARS AGO WOULD HAVE FOUND OUT AND BY NOW EVERYONE SHOULD BE 10Meters TALL
I mean, bringing Taoism or whatever into this,
its just trying to make it sound more convincing to stupid people
so I’ll tell u this zixia
I’d rather pay you 5 bucks to GET THE f*** out of this forum than to make you convince me that sitting down closing my eyes and imagine that I would taller.
Maybe I would get taller
IN MY DREAMS
so once again F*** you and f*** off
bye
|
|
| Pepe_Deluxe |
Call that a fair trial?
|
September 12 2006, 11:10 AM |
Basically the scene here looks like Zixialin has been rounded on by a pack of wolves.Okay, many of you are sick to death of scammers, and don’t want to be led by the nose into giving cash for something with no more substance than dreams.
I understand that completely. There are many cut-throats out there who deserve a beating for what they try to make off vulnerable people, but are you sure this is the case here..?
Zixialin is only asking for a ‘fee’ if people want to be personally guided by her. How many martial artist instructors, or even meditation class tutors, have you heard of that offer classes for zero money…? Are they scammers too because they value their time?
Many charity workers get some kind of pay, because bills need to get paid no matter how kind or ‘spiritual’ people are!
If you check her site, she’s giving you the core of the technique away for nothing.
Why not at least try the method, before aggressively lashing out and insulting her? No wonder she’s packed her bags – she isn’t obliged to take that kind of b/s off anyone. Why would she want to help if all she gets back is spite?
Have a look at this clip of what may be a genuine master of the energies the Taoists discuss :
http://www.devilducky.com/media/44318/
“There are stranger things in this world…”
|
|
| SD2002 |
There’s a huge difference
|
September 12 2006, 1:20 PM |
|
Instructors get paid because they have the goods and have ESTABLISHED themselves. This person hasn’t. All she’s saying is to use your mind power and she’ll help you for a fee. We don’t know if she’s legit or not. Martial arts instructors usually list their credentials and former teachers so you know what you’re getting into. All zlisjlksfj did was say she follows Taoism (which she doesn’t because you don’t follow Taoism to begin with) and that Qi Gong helped make her grow taller.Anyone else find it strange that people who’ve never/rarely posted on these boards before come and defend these strangers?
|
|
| ed81 |
Daoism and HI
|
September 12 2006, 1:52 PM |
|
Some ppl (scammers,of course) seem to think that this is a forum of imbeciles.The next one probably will present a topic about Daoism and HI.
|
|
| brother_komodo |
Re: Just for those who have interest in my method
|
September 12 2006, 6:24 PM |
|
maybe she shouldn’t have mentioned that she charged 5$ she should have probably given free trials first and THEN started charging once some ppl have grown
|
|
| Pepe_Deluxe |
Yes, and there’s a huge difference in what’s being charged too
|
September 13 2006, 10:54 AM |
SD2002:I doubt many dojos/meditation centres would give you a year’s membership for the equivalent of 18 dollars.
Just because someone has no stated formal qualifications doesn’t equate to ignorance. She claims she has studied Taoism (almost obsessively) for many years. Do you know enough about the philosophy to dismiss her so offhandedly? Check here and see if you do:
http://en.wikipedia.org/wiki/Taoism
Your point that she uses the word ‘follows’ implies she’s a scammer is ludicrous; you should be bearing in mind that English isn’t her first language and cut her some slack.
Also purposely mis-typing her name, and generally being very rude when she has only been polite and civil reflects badly on you.
“We don’t know if she’s legit or not.”
Exactly, so why treat her as guilty before you’ve tried a single thing?
I’m not endorsing her method at all, or encouraging people to get up (possibly) false hopes. It could be a swindle, though it would be a very strange one. Also, there’s the possibility she’s mistaken about her height gain (or that it’s somehow come about by some very rare physiological factors); the mind can easily play tricks us sometimes, especially when it comes to the things we want the most.
However, the people on this forum could be here for years trying to find a way to grow taller, so for your own sakes it makes sense to give everything remotely plausible a fighting chance.
Interesting that no-one has so far commented on the clip I linked to. Why’s that I wonder..?
Inexplicable things don’t just happen in the realms of ‘mysticism’ either. Aspects of quantum physics can’t be logically explained, yet nevertheless the theory is almost universally accepted by scientists.
This is a video description of the so-called ‘Observer Effect’, which summed up, basically means that the behaviour of particles can be changed simply by looking at them. :
http://www.whatthebleep.com/trailer/doubleslit.wm.low.html
The scientific community doesn’t know how or why it happens, but that doesn’t stop it from happening.
|
|
| ed81 |
Pepe_Deluxe
|
September 13 2006, 2:18 PM |
I have downloaded this movie from internet “what the bleep do we know”
also the next “down into the rabbit-hole”about quantum-physics,as you know very interesting but it also is a lot of speculating maybe over twenty years scientists have again other theroties. Being a black belt full contact karate-practioner (Kyokushinkai-Karate)
i once in a demonstration of a Tai-Chi master was swept away for 6 M
with a slight touch of his hand,although very amazing but these still are laws of nature,it was a nice guy he explained to me that through years of meditation he was capable of doing this by controlling his qi,(universal energy,present in everything).I agree with SD2002 out of the blue a girls enters this board with some vaque pronouncements about Taoism we know nothing about her credentials and she start charging a certain amount,c’mon man who will fall for this.
I happen to know something about Taoism and Daoism and the way she explained about Tao,the difference (in a thread to me) between rational thinking and intuition made her loose her credibility (IMO).Besides i think it is objectionable to charge money on a forum like this,if you really want to help them with some knowledge or whatever do it for free or leave.
|
|
| Zixia |
Re: Pepe_Deluxe
|
September 13 2006, 4:20 PM |
I am back for the saying “There are more than meets the eye”.
You just gave me your arbitrary conclusion with no further explanation. What is the “laws of nature” from the Taiji master that you understand so well? How absurd is it to you about my explanation? If Qi can push outer thing away without seen the Qi, why you can not understand it can perform inside? Have you ever practiced Qigong? Have you ever felt the move of your Qi? Have you ever sit in a class to listen to a Qigong teacher’s explanation?
Since you know something about Taoism, how many Taoists have your visited? How many mountains have you climbed in China? How many temples have your done research on? How many scriptures have you read? Why did Zhang Daoling start his Five Bushel Rice Taoist Sect? What happened to him? Why East Jin was full of Taoist pursuers? Why did Buddhism and Taoism have some many arguments and agreements? Why did Tang respect Taoism? Why did Quanzhen sect become so popular? How did Taoist Canon become into being? What is the central theme of Taoism? Taoism is abstract theories or it does care our normal people’s daily life and ultimate concern. Furthermore, why did Taoist scriptures full of discussion on the connection between our inner body and exterior world? What is the function of Sun and Moon to Taoist? What is the function of meditation in Taoism? What things Taoists claimed they can do in the scriptures?
How did I loose my credibility? Give me your explanation on what rational thinking and intuition is.
I just start spreading my story, and came across your board. I am not a fan or iron member of your board. I do not see anything wrong that I charge some for those who want to get my personal training. I will spend time and use what I have learned to guide people through their difficulties. I am not a fairy yet, you may now some economic rules. Oh, you are a Kyokushinkai-Karate, why don’t you send it to me since you are so generous. |
|
| ed81 |
zixia
|
September 13 2006, 5:23 PM |
First of all English is not my native language (i am from Europe),so sorry for the mistakes and i hope you understand everything i post.
Read my thread carefully please,the Tai-chi master who swept me away like a little kid (i am 90kg)explained to me that he just used the laws of nature,
he explained something about Qi to me,also that everybody is capable of doing this,but only by long meditation and exercise,so it only is achievable for a few ppl.I never posted that i understand very well how qi works,who really knows?,of course there is more then meets the eye,viruses,bacterium,electricity,magnetic fields etc but it all is part of nature allthough we are not always able to understand it all,like a famous scientists once said,the more we learn,the more we discover how little we know.About rational thinking and intuition,the reason why we call something happen by intuition is we cannot see the link between the cause and the result,i do not agree with you entirely,intuition it is a kind of knowing without a rational explanation,it comes from a far deeper level of your mind,it can manifest itself in a lot of ways,for instance it can give you a presentiment about dangerous situations(which i experienced once myself)
I practise Zen-meditation,because it gives me a good and relaxed feeling,
also some Qi Gong exercises (experimenting a bit),i noticed,i must admit,it gives me more energy and increases my vitality,whether i experienced my Qi flowing,sometimes i have weird but not unpleasant sensations in my body,
don’t know if that is a manifestation of Qi,maybe i need some guidance.
Later in another thread i will give comment on your questions about taoism and daoism,otherwise this thread is going to be very long.
One thing about Kyokushin-Karate i don’t understand your last phrase,what do you mean by sending it to me?????
|
|
| frans |
What is Tao?
|
September 13 2006, 8:48 PM |
There are two kinds of Tao and both Tao happens to be completely different. Though they happen to have only one English name which is Taoism.One is the Tao of Philosophy or “Tao jia/qia”(the pronounciation is a bit of both) and the other the Tao of Religion or “Tao Jiau”(Pronounced Tao Ji-A-u). Tao of philosophy and Tao of religion are names I created.
Tao of philosophy speaks on taking a larger world perspective rather than the narrow selfish human perspective. Its about giving up our own dominion towars the world, taking the stance that we are the world we live in rather than putting up a barrier between us and the world.
Tao of religion is about worshipping some demons, complicated rituals, ideas of afterlife etc. They are particularly fixed on methods of living forever or immortality. In chinese movie, they are the ones in weird yellow clothes, holding chimes in their hands and drinkin alcohol and spewing them onto candle. Of course, not all of you might have seen these.
Qi gong is unrelated to the above two Tao so Don’t mix them together again!! though they are often mistakenly taken to be the same…
|
|
| frans |
What is tao ?(2)
|
September 13 2006, 9:59 PM |
|
sorry I’m an idiot. I made a mistake.Qi gong is developed for Tao Of religion. it’s first initial purpose is for immortality.
|
|
| SD2002 |
Just read Lao Tzu
|
September 13 2006, 11:09 PM |
|
Everything else just makes Taoism seem too confusing. It’s exactly the opposite to what the Tao Te Ching means.
|
|
| Zixia |
Re: Just read Lao Tzu
|
September 14 2006, 12:38 PM |
|
So these people must waste their time on doing these, eh? http://daoiststudies.org
Since you are so smart, explain the first chapter to me.
|
|
| Zixia |
Re: zixia
|
September 14 2006, 12:50 PM |
In order to achieve anything valuable, you have to put effort on. But with better method, focus, personal willingness, the path can be quicker. A right teacher is important, that is my role for those who want to grow taller with my method, I can find out what the pitfalls they are in on time.”intuition it is a kind of knowing without a rational explanation,it comes from a far deeper level of your mind,it can manifest itself in a lot of ways,for instance it can give you a presentiment about dangerous situations”, you did not even explain what is called rational explanation, no a clear logic mind to see here. I could easily explain why you felt like that by my explanation.
You can either say Taoism or Daoism, there is no Taoism and Daoism. And you can not explain all of my questions in another thread since I wrote quite a few thesis on some of the questions.
After all I learned those ancient, modern practices, theories in order to find out a solution for myself: how to grow taller. I found it and that is it.
I mean your black belt, hohoho
|
|
| ed81 |
zixia,please reveal your secret to achieve HI (for free)
|
September 14 2006, 2:45 PM |
A rational explanation makes acting of human beings understandable from reason not influenced by a causal explanation.Is this understandable for you?There sure is something like Daoism and Taoism religious (without dogma’s or maybe with dogma’s)and philosophical way to look upon life in general (maybe the wisdom really comes from the East).There is a paralel between Quantum physics and Eastern philosophy.
Though you apparently got quite some knowledge about these subjects really doesn’t mean a thing,is it integrationed in your life?reading your threads i doubt it,can you control your Qi?,the way you illustrate your knowledge is the same as learning karate just out of a book.
Well,reveal your secret after your long and profound study to us how to achieve HI,being so anxious to help ppl,you without doubt will do it for free.
About my black belt,like the late Bruce Lee said “it only useful to keep up one’s trousers”,i have’n just learned to fight in the dojo but also in the streets and bar’s,being able to break some tiles and having a black belt is not that important allthough i must admit it is useful to master some karate in a fight (from my own experience and not in the gym)if you like want my belt put your adress on this board and i’ll send it to you.
|
|
| Pepe_Deluxe |
Science/Qi/Paranormal
|
September 14 2006, 6:09 PM |
ed81:Quantum theory may involve lots of speculation, but the strange, and seemingly impossible effects it throws out are certainly happening over and over in strict lab conditions.That’s why it’s the biggest foundation, even more so than standard classical (Newtonian) physics, of science right now.
It may turn out that there is yet another unseen force in addition to the four fundamental forces currently known (i.e. strong force; electromagnetic force; weak force; and gravitational force) that would account for particles having their properties and behaviour altered so mysteriously. Maybe there are several forces waiting to be uncovered. It might be the case that these unknown variables have something to do with our consciousness. And that would have strong implications for so-called ‘mind over matter’…
Who knows when that knowledge (whatever it turns out to be) will appear on the horizon, but the day will surely come.
Interesting story about the Tai Chi master who sent you flying. But as Zixia indicates – wouldn’t that by itself indicate a ‘bending’ of the accepted laws of nature? And if you can accept *that*, then why is accepting the possibility of affecting change within oneself (surely a more straightforward task than affecting someone *else*) too much to swallow?
I was doing a little digging on the ‘yin/yang master’, John Chang, shown in the very first link I put up here, and found this place:
http://www.wongkiewkit.com/forum/archive/index.php/t-289.html
There is someone calling themselves ‘darkrider’ who seems to be playing a subtle joke on everyone (quite funny in a way if you don’t take things too seriously), but things get a lot more interesting way down when ‘3rdlevelMoPai’ posts on the 10th August 2006. There’s a lot to wade through, and I’ve only read fragments, but it will be of interest to those curious about what advanced martial arts practitioners are alleged to be capable of doing with ‘Qi’.
I don’t know what you mean about “the way she explained about Tao, the difference (in a thread to me) between rational thinking and intuition made her loose her credibility (IMO).” Which post are you referring to?
Ok, you and others want the goods for free. Alright, I now see the forum does say in the small print that this place is supposed to be about free stuff, but hmmm….Zixia is giving away most of the method on her blog, and appears to be charging only for a refinement of some kind with guidance for a year bundled in. 18 dollars for several inches of height would be the sale of the century, but let’s ask her about this…
Zixia :
– Is it possible to grow any with what you’ve posted on your blog already?
– If so, what sort of time frame (period) for this growth could people expect?
– What finally ‘clicked’ when you were 22 after many years of practising Qi Gong etc, and why do you think it took so long to get to that point?
– Did other people remark to you that you looked taller? 3 inches in a year for a 22 yr old girl would be pretty hard to miss!
– How can you be confident it will work for others? Have you tried your method with anyone else you know?
I’m asking these sorts of questions in a friendly fashion of course, but stuff like this should be addressed as some people can get bitterly disappointed when they don’t get the type of results expected. Having hopes raised, then dashed against the rocks is a terrible experience.
Finally, here is a clip from Google Video that I found searching under ‘telekinesis’. Lots of way out stuff on this place – some of surely bogus, but some it…..hmmm….very peculiar indeed.
http://video.google.com/videoplay?docid=-4957790045041805261&q=telekinesis
I wonder if these young subjects are manipulating a force analagous to ‘Qi’? That’s assuming it’s not a clever fake…
|
|
| ed81 |
But so what?Just what is your point?
|
September 14 2006, 10:22 PM |
|
Is it so hard to read a thread,the Tai-Chi master himself admitted he used just laws of nature,it is not a matter of bending the laws of nature,but discover something new about nature and apply it,(if it allready was discovered thousands of years ago in another culture) or of a more recent period doesn’t matter.Same about Quantum theory,new theories,new discoveries from our point of view,but nature has always been the way it is,it will never change,human mankind will always try to understand it,this probably can go on for centuries and centuries.
But so what?Just what is your point?
|
|
| Pepe_Deluxe |
Re: But so what?Just what is your point?
|
September 15 2006, 4:23 AM |
|
My point was that what happened to you, i.e. that master using his ‘Qi’ resulting in you being “swept away for 6 M with a slight touch of his hand” would be labelled as ‘impossible’ by conventional thinking; just as using ‘Qi’ (or inner energy/whatever people want to call it) to ‘grow taller’ would be labelled as ‘impossible’ also. It should be pretty clear what I mean.Basically what I’m saying is, we don’t yet know enough about the ‘laws of nature’ to definitively rule out so-called impossible acts, and should therefore keep an open, but critical, mind.
|
|
| ed81 |
you have got a concept for growing taller Reveal it to us,same amount?
|
September 15 2006, 6:48 AM |
|
What is labelled as impossible by conventional thinking depends of the culture you live in amongst other’s,conventionel thinking is (IMO) not a standard.In China Qi is a well known phenomenon used to cure ppl (in their concept of medical science,of course)to improve’s one’s health and also it is just used in practising martial arts,but it (Qi is allready there)it’s proven,it doesn’t meet the eye but so does electricity.Of course nature still withholds a lot of secret’s for us,we always will keep trying to reveal those secrets,keep an open and critical mind also is a healthy mental attitude,what you really trying to make clear on this forum is that you have got a concept for growing taller derived from Eastern Philosophy just like Zixia,well reveal it to us.Same amount?
|
|
| Pepe_Deluxe |
I have a concept for growing taller and want to make money here? Eh, do I..?
|
September 15 2006, 11:53 AM |
Heh, you’ve made quite a quantum leap of assumption there haven’t you? Yes.If I wanted to sell a system on here, it’s bizarre I didn’t start my own thread and spoke out against the ill-treatment of a ‘competitor’.
No money sought here. No system. Me, I’m just curious to hear more details from Zixia. She doesn’t fit the profile of a typical scammer if you read between the lines. She writes as if she’s genuinely passionate about Taoism and how it relates to the potential of Qi energy, and probably has spent a lot more time than anyone on here studying and practising it. If it’s all a lie, it would be an incredibly devious one, and would go against the philosophy she says she’s grown up (no pun intended) with.
On ‘conventional thinking’. Well, let’s say ‘conventional thinking in the West’ then. However, would the majority of Eastern people necessarily believe your own experience with the Tai Chi master? I don’t know, maybe they would, but then maybe they would believe the ‘miraculous’ feats allegedly witnessed in other masters across the East, such as creating fire in bare hands, levitation, bullet stopping, and healing normally incurable conditions, were quite possible with the right amount of technique and practise too.
Where is the hard ‘proof’ of Qi? It can’t be scientifically isolated and used in homes like electricity to power your internet connection, but many would still swear it exists.
Maybe Zixia can show some quotes from Taoism/Qi Gong texts that lend support to the idea that the body can be altered using the kind of techniques she talks about. It seems far-fetched, but then I’ve seen things with my own eyes that would sound far-fetched.
I’m sceptical like you, but I’m open to being persuaded there might be something in all of this.
|
|
| ed81 |
if you have so much trust in Zixia’s method,well have a go for it
|
September 15 2006, 3:31 PM |
I allready told you about the Chinese concept of medical science,in China
you see in the early morning a lot of ppl practising Tai Chi,trying to harmonize their Qi flow for improving their health (allthough from origin Tai Chi is a martial art).Prove of The facts that Qi really works was once given by Chinese doctors to operate a patient without Narcosis,just using Acuapunture,Western medicans who whitnessed all this were astonished.
If you have so much trust in Zixia’s method,well have a go for it,let us know about the results.We are very curious.
|
|
| ed81 |
why so much trouble promoting Zixia’s method,are you two working together?
|
September 15 2006, 3:34 PM |
|
| Pepe_Deluxe |
Re: why so much trouble promoting Zixia’s method,are you two working together?
|
September 15 2006, 8:47 PM |
Yes, I admit it, we’re partners in crime here.Actually, we’re married and have a third child on the way, and to make sure both us and our beautiful, talented children live in the lap of luxury, we’ve scammed up half the net with the craziest **** you wouldn’t believe!
All of the fittings in our country estate dwelling are 24k gold, and we all have a personal assistants to cut our toenails. Even our cat (Frederico The Third) has a personal groomer and outfitter. The toilet is made of solid jade.
My real name is Rahul, and hers is Jennifer.
Now…
SHOW ME THE MONEY!!
Lol, paranoia seems to have got the better of you now it’d seem. I don’t know where you get promoting from. All I’ve done is point out that maybe there’s a chance Zixia isn’t spinning some fancy fairy tale just to make her shopping trips last longer.
If you’ve been paying attention, you’ll notice I’ve asked her some challenging questions in the hope we’ll all get a better handle on what’s happened with her. Thus far no-one has lost a single dollar here [well, no-ones complained about it if they have], so I find all the effort you are putting into giving her the bum rush (<slang for dismissal, for those who don’t know) a bit puzzling…
Especially since looking back I see you wrote this :
“but it is possible to achieve HI with other Qigong exercises, who knows,i won’t exclude it.”
Let her speak some more if she hasn’t been put off by all the negativity on display.
|
|
| ed81 |
no need to get so smart,ppl who really want to help doesn’t charge any money
|
September 16 2006, 2:51 PM |
No need to get so smart,it has nothing to do with paranoia,we have got a lot of bad experiences with scammers on this forum,i indeed wrote (i read that somewhere in a book about Qi Gong)and practised this exercise that a certain exercise named standing like a three,stretches the spinal column,collect your Qi in your Dan Tien,a area a couple of cm’s behind your navel,makes you stand firm and has a positive effect on your health,it however can take some years before any HI is achieved.(in my case nothing happened,allthough with my height (1.74) it is not a big problem for me)Standing firm was my goal.Zixia can speak as much as she want,but she must be able to take some critical remarks,if she cannot she not worth paying further attention to.
You have’n been put off by a critical approach,it is not meant personal,
forum-members always will be suspicious when somebody asks for money,on one hand Zixia is very anxious to help ppl with height problems,on the other hand she charges money for it that is a bit contradictory,don’t you think?
I won’t delete your posts because i agree with Joey’s (forum-owner) policy,
it wouldn’t be fair cause forum-members might be interested but personal
i think it is a lot of crap,somebody who really wants to help doesn’t charge any money.
|
|
| usa00 |
i agree with ed 81
|
September 16 2006, 5:33 PM |
why not just share everything for free. Too many people, have gone through too much frustration and wasted a lot of their hard earned money on crap.On the one hand I could understand wanting to make money and if u have some breakthrough product that guarantees people growth of a certain number of inches or something well then i can understand. But we’re talking about something that only zixalin has experienced results with, share with everyone else on here, then we’ll find out if it really works.
If one day i found the way to grow taller, i’d copywrite/patent it (so no one could profit from it monetarily) then id share it with everyone on the forum. That’s the honest to god’s truth. Ive been on these forusm too long. And its getting ridiculous with scammers and people popping on and saying i grew with my method, and not sharing any more details and then people asking a million questions and the guy (or girl) never comes back.
Zixalin just share it, how much money do u think ur actually gonna make? Ur just coming off as greedy.
|
|
| SD2002 |
They definitely are in cahoots
|
September 16 2006, 10:39 PM |
I’ve never seen Pepe on this board until zixia posted. And he’s ULTRA defensive of her. They try to play it to make us agree with their point of view (typical salesman gig) to try to get us to buy. At least Rahul gave people free trials.Like I said, I was critical of the way she was using Taoism in terms of height increase but she is probably more interested in the religious sect that spawned later that really doesn’t relate to the original philosophy. The problem is she’s acting like her method is guaranteed to make people grow taller, even though:
A: she doesn’t have any test subjects or loyal people to this board to practice on to see if she’s legit
B: doesn’t realize we can be doing this all for free, all she has to do is point us to reference sites.
C: she couldn’t handle the heat, leaves, then returns to CONTINUE to get us to believe.
Like I said, people on here have been scammed before and they don’t like being scammed over and over. Sales people who buck under the pressure the first time seem less credible. I believe that her methods COULD lead to height increase, but it’s all mental. I could meditate on growing taller and it would be just as effective than her method. I don’t like the way she’s charging for tips.
|
|
| Zixia |
I have no secret and all I say is
|
September 17 2006, 2:16 AM |
|
including Pepe, your questions are so silly since I answered them in my blog if you read. Well, except one, my friends commented my height a lot, especially my classmates in middle and high school. My parents were amazed by me. Some of my friends asked me how I did it, which was one of the reasons I finally thought to open a blog.
The reason I charge is so clear and I do not know why you people here are so fussy about it. How many people do you think I can guide in a year? If I have too many people come to ask me, I will raise the fee in fact.
Finally, I have no secret. I am gradually explain everything on my blog for you for free. The only little bit left is for those who want to further study with me. I put that part here in the past, what happened then? I need respect my own work at least a little bit.
I believe there are many ways to grow taller, mine is just one of many and you should again use as many as you can in order to get the result.
My method is not just for growing taller, if you keep on practicing, you will find this is a threshold for you to open the door to see the world you have never experienced. I will explain this in my blog later of course.
|
|
| ed81 |
including Pepe?i don;t think so,but…..
|
September 17 2006, 7:27 AM |
|
Are you as the Buddhist call it an enlightened person?As practioner of Zen i know you easily can be the victim of illusions created by your own mind,how beautiful they may be,but about your inner state of mind i cannot judge of that.But beside that Zixia,everybody reading your threads on this forum
will probably sent a thread to you and finding some candidates you can give it a go,i gave my opinion,but in fact (i must admit)my opinion is not that important,cause every forum-member has his (her’s)own responsibly and
can make their own decisions,so we’ll see how this develops.
|
|
| Jennifer Lynae |
ed81
|
September 17 2006, 2:53 PM |
|
You are extremely irritating? Why do you have to act as if you know everything and be so critical of others? I’m not saying this chinese girl is right, but at least please don’t give such assinine all-knowing condescending comments
|
|
| Craig Jordan |
hey ******* named jennifer lynae
|
September 17 2006, 3:30 PM |
|
You’re just a joker, we know you’re not the original jennifer lynae because of your damn ip address, you’re really pissing me and other forum members off. And please, if you’re going to use big words to put emphasis on how silly everything spell “asinine” right, will ya? Just get the **** off the damn forum, because you suck ass. I’m still waiting for your naked pics by the way, i don’t want them by the way, because i know you’re a guy masking yourself as this annoying girl. Ed could you please just get joey to ban *******s like jennifer lynae, she is a super bitch. This is the kind of stuff i’m talking about.
|
|
| Jennifer Lynae |
You too
|
September 17 2006, 3:39 PM |
|
You too should watch your mouth, instead of speaking in such a condescending manner just like an American who doesn’t watch her mouth. Just watch your mouth and don’t let it talk rot which doesn’t make sense
|
|
| ed81 |
why a questionmark behind irritating?
|
September 17 2006, 5:04 PM |
Read my threads well,Jinsee,i am just critical against ppl who want to make money on those forum,they also are critical against me,that is no problem for me,cause for clearness sake it is not that bad,again read all the threads well and stop moaning.My last post to Zixia was quit reasonable,allthough when Pepe stops posting,she(he)starts again,perfect timing (i don’t know who is who and i am not interested too),but this girl or boy can have a go on this forum if she (he) wants,if candidates are available,isn’t that marvellous?Don’t
hope too many forum-members will fall for this.
I agree with Craig,stop using the word assinine,it a bit out of proportion.
|
|
| SD2002 |
Zixia, either post pics of proof that you grew or take a hike
|
September 17 2006, 5:32 PM |
|
| ed81 |
hey ,Jinsee alias Jennifer
|
September 17 2006, 6:30 PM |
|
Hey jinsee alias Jennifer somebody on Frogger’s forum posted the url
of your web-site,i know what you look like (a young girl of sixteen years old,claims to be a twenty year old doctor from Singapore),i also know you are a very poetic girl,but why harassing this forum?Another question Kewl asked about your HI methods,why didn’t you give him a response?
|
|
| SD2002 |
Boards gone mad again!
|
September 17 2006, 7:37 PM |
|
First Rahul, now this! Homeopathy, vibration theory, qi gong, and I’m still 5’9!
|
|
| ed81 |
who knows what’s next,maybe Bungee jumping
|
September 17 2006, 8:19 PM |
|
| Craig Jordan |
dont you see
|
September 17 2006, 8:21 PM |
|
it is just another prankster venting their frustration out on innocent people in an tranquil forum, they do this type of stuff to stir up controversey. They are no worse than a bunch of people who commit terrible acts of crime, because they are insensitive and downright stupid. This jennifer lynae girl is one of the most unintelligent people i have ever had the displeasure of ever encountering, she only has a few words in her lexicon like “asinine” and “condescending” and she is totally nonsensical. I couldnt really tell you about the other one, because she sounds sincere, but she does look like she is using taoism and other -isms as a gimmick to attract people into thinking that it works better that way. Honestly ed81, it is better to delete this thread.
|
|
| ed81 |
craig
|
September 17 2006, 8:39 PM |
|
No, Zixia is the girl who claims she can make ppl grow with Qi Gong exercises she wrote a lot about Taoism, Jennifer also claims she has got some unique methods to achieve HI,but nobody really know what kind of methods(has something to do with the spine),there soon will come an evaluation according to her,Joey’s policy is to give everybody a fair chance,because there always might be some forum-members interested,it wouldn’t be fair and democratic towards them to delete the posts,i agree,but after a while when establishing the fact that it is not really a contribution to this forum,you still can delete the whole bunch.We’ll see what is going to happen.
|
|
| Pepe_Deluxe |
No need to get so touchy ed81!
|
September 18 2006, 2:17 PM |
Can’t you take a little mild humour? After all, you think it’s perfectly fine to go about firstly implying Zixia is a scammer on virtually no basis at all.THEN imply I’m deceptively adding to this thread in some bizarre round about way of making money.
THEN indicate I’m in league with Zixia and trying to con everyone too.
You mysteriously come to the latter conclusion by pointing out that one of us posts, then the other posts at some other time…. Is this some kind of new logical reasoning system I’ve yet to hear about? Makes zero sense to me!
All this finger pointing isn’t ‘personal’? You need to think more before you type is what I think.
“on one hand Zixia is very anxious to help ppl with height problems,on the other hand she charges money for it that is a bit contradictory,don’t you think?”
Wasn’t this clear the first time around? No, it is NOT contradictory. Plenty of people charge for a multitude of services, while still wanting to help others. This includes so-called ‘amateurs’/people with no formal qualifications too. Knowing how babyish and pestering people can be when they want something, she could easily spend up to half the day sat in front of the screen answering pointless mails if she offered individual guidance for free. Moreover, she’s kept telling you *she’s giving away the technique away for nothing in her journal updates*. Only 1 on 1 guidance comes with a price tag.
So gracious of you not to delete my posts when you’ve got absolutely no excuse to do that anyway – thanks 
SD2002:
You’re more in need of an attitude improving forum than one for height. Seriously, you are one obnoxious hombre, and clearly think you’re a lot smarter than you actually come across as.
“They try to play it to make us agree with their point of view (typical salesman gig) to try to get us to buy”
Gee, who would have thought that salesmen try to get people to buy things?? Look, here come the FBI – they are crying out for men like you dude!
Listen up ‘Mulder’ : Zixia has made it clear more than once she’s charging only for the nannying service, not the method [Jeez, how obtuse can people be…? To infinity it seems]. Me, I’m only interested in getting the truth out of this, and giving people a chance to be heard, instead of watching them ganged up on and bullied by rude, self-appointed ‘know-alls’. Did it occur to you that not everyone who visits here posts? That there may well be people out there too shy to write but want to hear more about the ideas people talk about?
Because you haven’t seen me before, that makes me ‘in’ on this ‘fraud’? Not that I have to explain myself to the likes of you, but I’m not in the other threads because mostly I’m not interested in sweating like a pig for a year to get half a cm to be excited about, or paying hundreds of dollars for dodgy sounding, non-safety passed powder from strange sounding people on the other side of the world.
You’d think you owned this place by the way you act.
As for Craig Jordan, I’m not even going to bother apart from saying I hope for his sake he’s no more than 14, because that’s how old he sounds.
Guess you ‘clued-up’ guys reckon even the moon landings were a big conspiracy, eh? Maybe even that the world is secretly ruled by super-advanced E.T. intelligent lizards posing as humans too…
Seriously, ease off on the weed or whatever else it is that’s causing this laughable borderline pathological suspicion – it’s getting very stale now.
Zixia:
You include me in those asking ‘silly questions’, eh? Well, so much for my being “ULTRA defensive” of you!
I don’t see what you mean though. Thought I was being reasonable.
To recap with added explanation:
Q. “Is it possible to grow any with what you’ve posted on your blog already?”
Ok, there is a part in your blog that says,
‘No matter how old you are, measure your height, then follow my simple and unique way of breath for half an hour, then measure your height again. I will prove you that you will grow taller a little bit in this half an hour.’
A little bit can’t be counted, since small discrepancies in measuring will surely arise.
________________________
Q.”If so, what sort of time frame (period) for this growth could people expect?”
Yes, you describe your growth, but as you point out, you’d ‘built up’ your Qi level for many years. Most here will have no such background.
________________________
Q. “What finally ‘clicked’ when you were 22 after many years of practising Qi Gong etc, and why do you think it took so long to get to that point?”
Well, I checked again, and I can’t really see any indication of why it suddenly all fell into place in just 1 year.
________________________
Q.”How can you be confident it will work for others? Have you tried your method with anyone else you know?”
A reasonable question! It might have been useful to trial it on someone else in your life before going public, just to be absolutely sure it wasn’t some big-time weirdo coincidence.
________________________
|
|
| ed81 |
Aren’t you a bit rattling on?Well i hope you feel better now.
|
September 18 2006, 4:38 PM |
|
You better leave this forum,don’t know what you are up to (what is exactly your contribution to this forum,still haven’t find out yet)critizing everybody,but gradually you are getting a bit tiresome,creating a lot of anxiety on this board,irritating ppl with silly remarks about ET intelligent lizards etc,it’s all a bit childish,this thread reveals more about you then other forum-members if you go on like this you better take a hike.
|
|
| ed81 |
you are insulting other forum-members, with remarks like ease of the weed
|
September 18 2006, 6:37 PM |
|
Ease of the weed is quit insulting,(i overread it at first)you can apologize for that,if not,then it was your last post.
|
|
| Zixia |
Pepe
|
September 19 2006, 10:58 PM |
I have been busy and no time to check this board in the last few days. I admit that I should not include you in those asking “silly questions”.Q. “Is it possible to grow any with what you’ve posted on your blog already?”
A. It has been proven again and again in Qigong class I studied, age was not a problem to
have one’s finger to grow longer in a few mintues. So I conclude people can grow taller, effort, time, and determination are the matter.
Q.”If so, what sort of time frame (period) for this growth could people expect?”
A. You are right, my background is different from those with no knowledge about Qigong. But I still believe one year is a good time frame. People’s nature is different, some can grow faster than others, and the background is not that important. I will explain this in my blog later.
Q. “What finally ‘clicked’ when you were 22 after many years of practising Qi Gong etc, and why do you think it took so long to get to that point?”
A. I graduated from college in 2002 and moved to another city. I felt everything was fresh and I need a real life in the new year.
Q.”How can you be confident it will work for others? Have you tried your method with anyone else you know?”
A. To be simple, the confident is from the idea that human beings are guided by our mind, only because our mind is fixed, our body is fixed. Once our mind changes, our body will follow. I have not tried with anyone else completely.
I am posting my method on my blog, anyone can try it from reading it. There is no coincidence, I see the cause and effect.
BTW, ed81 claimed himself as practioner of Zen and know I easily can be the victim of illusions created by my own mind. First of all, I like to hear how Zen Buddhism defines what illusion is. Then you will find out what I have said does not fit that catogory. And I do not like you to label me as being in illusion by guessing what I may say or think.
|
|
| ed81 |
zixia
|
September 20 2006, 4:20 AM |
Being a practioner of Zen is nothing special everbody can do it,you just want definitions of everything,problem with you is you are (IMO)much too rational (that is exact what most Eastern philosophies reject).(by the way Zen is very hard to describe,but very simple to practise,but also very difficult)Again read my thread well,i also posted “but about your inner state of mind,
i cannot judge of that”,didn’t you read that?
Gradually it is time to reveal something of your blog,as you posted before,
we all are very curious.
Of course when the mind changes the body will follow, but to apply this on such a complicated bio-chemical process of growing,a lot is possible but beyond certain limits,for instance ppl in a hypnotic trance can do unbelievable things,but not get taller.
|
|
| brother_komodo |
hey
|
September 20 2006, 7:50 AM |
|
does anyone here follow harry potter?? Cos zixia remidns me of Professor Trelawney (the stupid divination teacher ). Nothing on you of course zixia but just the way you talk I find it funny no offense.Just a random thought
|
|
| ed81 |
Sorry Zixia,forgot to answer you question in the wright way
|
September 20 2006, 9:55 AM |
|
Illusion is the blindness to be imprisoned by what one considers as the truth,in this state of mind one will not be susceptible anymore,Zen is all about awareness and susceptibility.(always keep an open mind).
|
|
| meneka |
to ed81. urgent!!
|
September 20 2006, 4:33 PM |
|
hi. im female 5ft 2, u have given me some great advise before and was wondering if you could help me out in this one. seen as i am short i always wear high heels. i know in the long term it is not good for a womens posture, however do u think it still could be possible for me to gain at least an inch in 1 year from doing stretches, but still wearing heels when im out and about. i know you’re not a woman but u are so knowledgable thats why i thought if i could ask you.
please reply back
thanks
|
|
| ed81 |
Re: to ed81. urgent!!
|
September 20 2006, 6:35 PM |
|
How much height somebody will gain after doing stretching exercises is alas not predictable,it differs from person to person,even genes play a role.(i take it your bones are fused).
The only thing i can advise you is go for it,experiment with different methods like Yoga,stretching,Inversion or hanging from a bar,i just read a post from SD2002 that Pilatus can be very effective too,i daily take Glucosamine and chondroitine,also read the other threads for some good tips,and take a look at www.easyheight.com.Good luck with it.(it is not bad for a woman to be short,my wife is not much taller,or am i wrong?).
|
|
| SD2002 |
Yeah do Inversion, hang from a bar, Yamuna, sulfate, ect
|
September 21 2006, 12:04 AM |
|
We’ve posted a lot of stories of people growing from all these things. Check out www.easyheight.com for more on inversion which should get you inspired. Short girls = sexy, why oh why do ladies want to be so tall?!?
|
|
| Zixia |
ed81
|
September 21 2006, 12:33 AM |
First of all, if you check carefully either Zen Buddhism, Pure Land Buddhism, Huayan Buddhism, Taoism, Confucianism. To have a clear definition on what your are talking about is one of the most important things in each religion or philosophy. Otherwise, we can not continue our conversation since we may talk about different things even though we use the same term. Of course the definition is not the way we usually use as after Industrial Revolution time.
Second, what you said what illusion quite different from Zen Buddhism, you may see here:Bonno (Japanese) desires, illusion; mental functions which trouble the mind, passions, false views. “Desires are natural; they become bonno when there is attachment.” (Deshimaru, Questions to a Zen Master). “Desire itself is natural and is harmful or misleading only when we cling to or resist it.” (Deshimaru, The Zen Way to the Martial Arts). (You can keep on reading more, and more terms here, hehe http://www.yakrider.com/Buddha/Zen/zen_terms.htm)
For our agreement, here is more to read for you if you have not read what illusion is in Zen. http://sped2work.tripod.com/AllThingsZen.html
(Well, I am not a total web girl. I can give you a booklist on Zen Buddhism if you need.)
Illusion and Tao have something similar that is Tao is everywhere, therefore you can actually find it and do what you want, on the other hand, we are in illusion therefore once we find the causation we can make the difference.
I am not that rational and do not need you to explain every term. But what you said does not have solid ground, and most likely is from your assumed Zen thinking.
Second, since you can not judge my inner state of mind, I suspect you can say that I may be in illusion. Especially I said that my method is a threshold for more things to find out. I have not written on this point yet.
Either getting taller by my method or not is not guaranteed to everyone, but I see the potential to everyone. That is why I am writing more on my blog for you to judge.
|
|
| ed81 |
short girls
|
September 21 2006, 7:22 AM |
|
Yeah,even if i would be very tall,i always will have a weak spot for short girls,i even don’t like tall girls,but okay everyone to his taste.
|
|
| ed81 |
zixia
|
September 21 2006, 8:32 AM |
Let’s forget for a while the whole story of the Chinese or Japanese historical background,different schools etc,because it all comes back to the same:Also a little story: A man from coming out of the crowd asked a Zenmaster Ikkyu: What is the greatest wisdom?,the Zen-master wrote down Awareness,the man asked :Only that?,don’t you want to add something? The zen-master again wrote down and now two times awareness,The man got agitated and spoke;i cannot discover not much wisdom in what you keep writing down,the Zen-master again wrote down now three times awareness,the man now desperate
called:but what does awareness mean?The Zen-master friendly answered:Awareness means awareness.For me this story was quit revealing,cause real awareness as practised in Zen-meditation is really the key,in a way it has nothing to do with Buddhism,Christianity,it gives more insight in the way we function with our attachments,fears joys,etc from that insight (sometimes)we can obtain spiritual freedom,a Koan is useful because it is meant to clear away all existing ways thinking-patterns and expectations,the solution is not in rational thinking,so after a while students dicover from a new insight that there are no real hiding places in the spirit.In furthers stages pupils are often referred by their master like:forget Zen,forget Buddha forget everything,all these thinking-patterns about these subjects all inhibit the spiritual development,i can understand this now.
Thanks for the offer,but i allready read a lot of books about Zen (before i started) (Mr Suzuki)amongst other’s,about Zen-Buddhism,also read Krishnamurti,i read about Occultism,Ayurveda,i am just
interested in a lot of things,also in how to hack a computer,but that is quit something else.
One question,with your knowledge,insight,how can being a bit on the short side disturb you,one would think at your level of mental development it
doesn’t play a role anymore.For a lot of men(including myself)short girls are charming).
|
|
| Joey |
Zixia
|
September 22 2006, 12:21 AM |
Pepe wanted to send you a question, so I’m gonna re-post it myself so you can answer him.=======
>
> Zixia:
>
> Good to see my questions don’t look so silly the 2nd
> time around!
>
> “A. It has been proven again and again in Qigong
> class I studied, age was
> not a problem to have one’s finger to grow longer in
> a few minutes. So I
> conclude people can grow taller, effort, time, and
> determination are the
> matter.”
>
> Hmm, forgive me but this seems really weird.
>
> Firstly, it seems highly unusual that such a
> significant change in actual
> bone structure could be affected in virtually no
> time at all. I
> mean…*minutes*..?
>
> Secondly, that almost everyone in that group you
> mentioned managed the same
> effect without trouble.
>
> Thirdly…why on Earth would anyone want just one
> finger to grow much longer
> than their other digits permanently..? It’s not
> something I’d be happy to go
> around with! Or was it a temporary effect?
>
> Were tape measures brought out to verify the change?
> Just putting one’s
> hands together wouldn’t prove much unless you’re
> talking like an inch plus!
>
> And if it was possible to sprout extra-long fingers
> in just a few minutes,
> shouldn’t it also be possible to grow overall
> length, i.e. height in a very
> short time too?
>
> ____________________
>
>
> “A. I graduated from college in 2002 and moved to
> another city. I felt
> everything was fresh and I need a real life in the
> new year.”
>
> Well ok, but according to your journal you had very
> strong motivation for a
> long time before the ‘revolution’ period. It seems
> unusual that you got
> little in the way of observable results for all
> those years, then suddenly
> WHOOSH, 3 inches in 12 months!
>
> What insight into Qi was so powerful it managed to
> put you into
> ‘turbocharged’ mode? Perhaps you could show some
> text from the ‘Dao De Jing’
> book to highlight what reading this did to make such
> a dramatic leap
> forward.
>
> On a side-note, the site you link to that contains
> Taoism books will only
> let you join and read the online books if members
> agree to contribute
> something academic…I’m no Taoist scholar!
>
> _____________________
>
> Also, looking at the notes in your blog, I can’t see
> any difference between
> the 1st and 2nd steps! Am I missing something here?
>
> =======
>
____________________
Also(this is coming from me myself) I’m kinda looking at your blog a bit more and waiting to see what updates you make.
|
|
| usa00 |
yeah same with joey and pepe
|
September 22 2006, 11:50 AM |
|
interested, in permanency, how much the finger grew, etc. can you do this with feet/toes?
|
|
| ed81 |
finger growing in a couple of minutes,c’mon,is this board going mad?
|
September 22 2006, 12:17 PM |
|
Some body-parts can,but not your finger.SD2002 is right this forum is going the wrong way.I once posted after Rahul just for fun,what’s next,but never dreamt of this.
|
|
| Zixia |
Re: zixia
|
September 23 2006, 2:49 AM |
ed81, finger growing is true and only when you were there in class, could you be quietly accept it, it was just so easy. Unlike people here looking for HI, most people I encounter do not care their height in Qigong class, they want to become healthier, smarter, or more spiritual. I don’t really know what they want, but to have one’s finger grow is definitely something piece of cake for the beginners. Ask someone who was in Qigong class in China before. My words are surely not enough. But I certainly got my confidence from the experience that I could grow taller with the same idea then.Also ed, let’s look at your answers to me.
You said: I may be in illusion from your Zen practitioner’s perspective.
I said: No, I am not, since Zen’s idea of illusion does not fit me, namely, I am not attached to my
height problem. Illusion is not something bad or good to Zen as you know.
Then you said: What awareness is.
What you mentioned is one of Zen schools, sudden awareness. And the other one is gradual
awareness. Anyway, that was not my question. You did not answer my question: what is illusion? I answered it by Zen’s own words. Zen is heavily related to meditation, but it does not mean we can not clarify what we are doing by words, even though most time the words do not have rational connections. Otherwise, they really should toss their scriptures completely.
I sincerely worry about your moral stand, how to hack a computer is not something I would be proud to tell others.
Am I a tall girl now, btw ? huhuhu
|
|
| Zixia |
My answers to Joey
|
September 23 2006, 2:50 AM |
We tried an index finger, sometimes later the whole right hand fingers. No tapes, but it was so clear that our right hand fingers much longer than the left ones. I was actually scared at the time that my fingers would stay longer than the left side, but they turned to be the same. Without long period of practice, the sudden change will not be stable.Yes, it is true that it is hardly to believe that all people had their fingers extended in a few minutes without age difference. All I remember that people around me all made it.
With the same idea that we could grow taller in a few minutes, but through my own practice, it was much harder than we thought. But the other reason is, from Qigong point of view, where you practice it. When you are in a class with a lot of people with the same idea, the effect is much quicker since we have a very harmonious atmosphere and the Qi is strong. If home alone, we do not get the effect so easy.
There was a Qigong school teaching people how to grow taller when I was in China around 1998. I read an article saying that the school was full of short people wishing to grow a few centimeters, and the effect sounded quite good. I wanted to go there so much. But I did not have time and could not afford like half a year’s fee at the time. I bought their materials, a lot of physical stretches with mind guidance.
As I said, Qigong was not something in China to make people grow taller. It was introduced to let people be aware of their own mental power for health, wisdom. Unlike people here all concern about your height. In a Qigong class, children wanted to be smarter, middle age people wanted to be healthier, relaxed, senior people wanted to be away from illness. And a lot of people had their super spiritual experience.
If you were in China, you would know how stressful in high school in order to pass the college entrance exams. I had some free time in college, but spent a lot studying with different Qigong teachers. I was so interested in the whole system of Qigong and spent a lot of time reading as well. Since I believed age was not a problem, I was not in a hurry to increase my height then. And I was so tired at night with full schedule during the day that I went to sleep right away…xixixi
Read the first 37 chapters of Daodejing, not many words, I do not have time to point each word for you. While reading with growing taller method in you mind, you may find something interesting. I can highlight words for you later if you still want to. Too late tonight.
You are right, I did not explain my two steps clearly. The first step is to imagine the bright ball absorbing the energy from the universe. The second step is in a narrower and concrete way: to inhale energy to the ball. The path is more specific. I should point this out.
Well, you can still find a lot information from the daoiststudies website as a beginner.
|
|
| ed81 |
zixia
|
September 23 2006, 4:04 AM |
I agree with Joey,it is hard to imagine changing the bonestructure of a finger in a couple of minutes,it probably is a matter of optical illusion.Qi gong masters indeed can perform things average ppl can only dream of,
i know,but after long years of training,suppose there is a possibility through means of Qi Gong to get taller (not stretching the spine like in standing meditation)but really influence the very complicated bio-chemical process of getting taller,just hypothetical,you (IMO)seem to be a bit too young to have such skills.
Reading your blog i noticed a similarity between Magic rituals (Occultism) also visualizing bright light floating through the body,inhale and exhale exercises,but then in different colours (depends what you want to achieve with it) experience the sensations,from the ball full of energy in this case above your head,it is recognisable,Qi also is guided with one’s mind.
Of course we should clarify in words what we are doing,i agree.
You don’t have to worry about my moral standard,haven’t got one (lol),
no Zixia,i am interested in how to hack computers,i read and study a lot about it,but that doesn’t mean i am going to hack a computer.that is qute something else.
If you understand this ,you really are a tall girl.
|
|
| meneka |
to ed81
|
September 23 2006, 6:53 PM |
|
i was just wondering that people say u should eat 2 hrs b4 bedtime. But before i go to sleep im hungry, if i had a glass of milk would that be ok for my body to preduce the sufficient amount of growth hormone whilst im sleeping. i was wondering if u ate before bed when u grew 2cms and what u ate? thanks
|
|
| ed81 |
meneka
|
September 23 2006, 9:06 PM |
If your food-pattern is sufficient and healthy during the day,there is no need to eat before sleeping time for getting taller.(even when you are hungry).In the morning the only thing i like is coffee,so i have to force myself to eat something,mostly porridge,before going to bed i am always very hungry, often i ate peanuts,cheese chocolat,etc,drank a couple of beers,but i stopped with that cause it increased my bodyweight dramaticly,only now eat a banana or an apple and a glass of milk,it also is much healthier.Having much appetite at certain times all has to do with the level of Glucose in your blood,eating regurlarly and healthy during the day,in a way stabilizes your Glucose-level,that is sufficient,it is not really necessary to eat before bed-time,it also is not a condition for growing taller,but i know it is difficult not to do it.
A healthy diet,combined with sufficient exercise will create the optimum conditions to produce enough HGH,but again this differs from person to person.
|
|
| meneka |
to ed81
|
September 24 2006, 6:52 AM |
great!! thanks alot ed81, ur always a great help!!  |
|
| ed81 |
meneka
|
September 24 2006, 7:26 AM |
|
One thing,Meneka,i think that (in my case) performing some Yoga postures,(Hatha Yoga),improved my own posture,decreased some light curvatures of the spine,maybe that is the reason of growing two cm’s,i am not sure whether thickening of the spinal discs played a role in it.
|
|
| Anonymous |
Heh, all those questions were mine not Joey’s but the answers came, so nevermind
|
September 24 2006, 11:16 AM |
I’m not sure what exactly might have been happening to suddenly extend all the participants bones, then shrink them again just as abruptly. If I was being cynical I would say it was possibly something akin to mass hysteria (or, group mind thinking), where rationality breaks down and people think and act as they think the majority should…I can’t say though – I wasn’t there after all, and you sound like you were in no doubt something took place.
The 3 inches in a year is less easy to explain away. There would have to be a massive distortion of perception for both you and those around you, to believe you had grown so unmistakably. If it was just you believing it, then a sceptic may say possibly a breakdown in your mind had occurred; perhaps schizophrenia or similar…Yet…you don’t sound mad!
Often, people think they’ve grown, when really they’ve just measured wrongly – it’s stupidly difficult to measure oneself accurately. However the 4 inches in total you say you’ve gained is shocking, and to make this much of a Godzilla-sized mistake someone would have to be off the scale incompetent.
I guess that all that remains is for people to try what you’re suggesting in your journal. At the moment it does sound almost identical to the concept of ‘creative visualisation’, which I have to say hasn’t yielded good results for me in the past.
Regardless, I’d say give all this the benefit of the doubt and see if anything happens.
Wouldn’t it be funny if the visitors here zoomed up to epic proportions from this…the board would close down! I’m sure some would overdo it, end up 9 ft tall and completely smash the record books, lol.
|
|
| Pepe_Deluxe |
Heh, all those questions were mine not Joey’s but the answers came, so nevermind
|
September 24 2006, 11:19 AM |
I’m not sure what exactly might have been happening to suddenly extend all the participants’ bones, then shrink them again just as abruptly. If I was being cynical I would say it was possibly something akin to mass hysteria (or, group mind thinking), where rationality breaks down and people think and act as they think the majority should…I can’t say though – I wasn’t there after all, and you sound like you were in no doubt something took place.
The 3 inches in a year is less easy to explain away. There would have to be a massive distortion of perception for both you and those around you, to believe you had grown so unmistakably. If it was just you believing it, then a sceptic may say possibly a breakdown in your mind had occurred; perhaps schizophrenia or similar…Yet…you don’t sound mad!
Often, people think they’ve grown, when really they’ve just measured wrongly – it’s stupidly difficult to measure oneself accurately. However the 4 inches in total you say you’ve gained is shocking, and to make this much of a Godzilla-sized mistake someone would have to be off the scale incompetent.
I guess that all that remains is for people to try what you’re suggesting in your journal. At the moment it does sound almost identical to the concept of ‘creative visualisation’, which I have to say hasn’t yielded good results for me in the past.
Regardless, I’d say give all this the benefit of the doubt and see if anything happens.
Wouldn’t it be funny if the visitors here zoomed up to epic proportions from this…the board would close down! I’m sure some would overdo it, end up 9 ft tall and completely smash the record books, lol.
|
|
|
| Current Topic – Just for those who have interest in my method |
|
Respond to this message |
|
| << Previous Topic | Next Topic >> |
Return to Main Page of Forum |
|
![]()
![]() (I wrote about Nicole as a biohacker.)
(I wrote about Nicole as a biohacker.)![]()
![]()