Along with the biohackers movement is another underground phenomenon that is getting a lot of attention these days. This movement involves a group of people trying to find the most cutting edge science and technology to try to extend their lifespan. For some, they would prefer to be immortal. In front of the movement is biochemist Bill Andrews, who I had first read about two years ago when I picked up a Time (or some other very big name) magazine from a Barnes & Noble in Seattle. Imagine my surprise when I found his name and research also talked about in a weekly college newspaper editorial from a top ranked university in Seoul last year. Then I see his name appear again on the podcast with Dave Asprey and I realized that I wanted to know about this guy, and his mission to find a way towards immortality.
This is the article/ story I managed to find from the Popular Sciences website. Again, I will highlight the parts which I found the most interesting and worth looking over.
The Man Who Would Stop Time

Bill Andrews’s feet are so large, he tells me, that back when he was 20 he was able to break the Southern California barefoot-waterskiing distance record the first time he put skin to water. Then he got ambitious and went for the world speed record. When the towrope broke at 80 mph, he says, “they pulled me out of the water on a stretcher.”
The soles of the size-15 New Balances that today shelter those impressive feet strike a steady clap-clap on the macadam as Andrews and I lope down a path along the Truckee River that takes us away from the clutter of cut-rate casino hotels, strip malls and highway exit ramps that is downtown Reno, Nevada. Andrews, 59, is a lean 6-foot-3 and wears a close-cropped salt-and-pepper Vandyke and, for today’s outing, a silver running jacket, nicely completing a package that suggests a Right Stuff–era astronaut. He is in fact one of the better ultramarathoners in America. I am an out-of-shape former occasional runner, so it gives me pause to listen as Andrews describes his racing exploits. “I can run 100 miles, finish, turn around, and meet friends of mine on the course who are still coming in,” he says. “I’ve been in many races where I’m stepping over bodies of people who have collapsed, and I’m feeling great.”
“I want to cure my aging, my friends’ and family’s aging, my investors’ aging, and I want to make a ton of money,” Andrews says. His return to running after a middle-aged break was, he says, inspired by a revelation he had at a time when he and a small team of scientists at his biotech start-up, Sierra Sciences, had been working 14 to 18 hours a day in the lab for five years, rather obsessively pursuing a particular breakthrough. Finally, his doctor told him he was headed for an early grave. “I thought, god, I don’t want to cure aging and then drop dead,” Andrews says.
That would indeed be ironic. Because Andrews does intend to cure aging. This stated ambition induces in some listeners the suspicion that Andrews might suffer from delusions of grandeur, but he has a scientific pedigree that insists he be taken seriously. Unlike his friend Aubrey de Grey, the University of Cambridge longevity theorist who relentlessly generates media attention with speculations that straddle the border between science and science fiction, Andrews is an actual research scientist, a top-drawer molecular biologist.
In the 1990s, as the director of molecular biology at the Bay Area biotech firm Geron, Andrews helped lead a team of researchers that, in alliance with a lab at the University of Colorado, just barely beat out the Massachusetts Institute of Technology in a furious, near-decade-long race to identify the human telomerase gene. That this basic science took on the trappings of a frenzied Great Race is a testament to the biological preciousness of telomerase, an enzyme that maintains the ends of our cells’ chromosomes, called telomeres. Telomeres get shorter each time a cell divides, and when they get too short the cell can no longer make fresh copies of itself. If we live long enough, the tissues and organ systems that depend on continued cell replication begin to falter: The skin sags, the internal organs grow slack, the immune-system response weakens such that the next chest cold could be our last. But what if we could induce our bodies to express more telomerase? We’ll see, because that is what Andrews intends to do.
Andrews had scheduled this afternoon’s run as an 18-miler, but he graciously downscaled those ambitions on my behalf long before we set out from the parking lot of the Grand Sierra Resort Hotel. Four miles in, he’s hardly winded—and I’m out of gas. As we make our way back to his car, he consults his training watch and informs me that our pace was an almost respectable 8:40, excepting the latter stretches when I walked, pushing our average up to 10 minutes a mile.
The embrace of fitness has for Andrews a telomeric logic. Make poor lifestyle choices, and you’re likely to die of heart disease or cancer or something well before your telomeres would otherwise become life-threateningly short. But for the aerobicized Andrews, for anyone who takes reasonable care of himself, a drug that activates telomerase might slow down the baseline rate at which the body falls apart. Andrews likens the underlying causes of aging, free radicals and the rest, to sticks of dynamite, with truncated telomeres being the stick with the shortest fuse. “I believe there’s a really good chance that if we defuse that stick,” he says, “and the person doesn’t smoke and doesn’t get obese, it wouldn’t be surprising if they lived to be 150 years old. That means they’re going to have 50 more years to be around when somebody solves the other aging problems.”
But in his race to cure aging, Andrews may himself be running out of time. The stock-market crash of 2008 nearly wiped out two investors who had until then been his primary funders. Without the money to continue refining the nearly 40 telomerase-activating chemicals he and his team had already discovered, Andrews made the decision last September to cut a deal with John W. Anderson, the founder of Isagenix, an Arizona-based “network marketing” supplement company. This month, Isagenix will launch an anti-aging product containing several natural compounds that Sierra Sciences has verified to have “telomere-supporting” properties. It’s not the powerful drug Andrews originally envisioned, but he says he believes it will promote “health and well-being” and just possibly generate enough cash to underwrite the expensive “medicinal chemistry” required to come up with a more fully developed anti-aging compound—one attractive enough to bring in a billionaire or a Big Pharma partner with pockets deep enough to take a drug candidate through the FDA’s time-consuming and fabulously expensive approval process.
“I want to cure my aging,” Andrews tells me, “my friends’ and family’s aging, my investors’ aging, their friends’ and families’ aging, and make a ton of money. And I want to cure everybody else’s aging too—I put that probably equal to making a ton of money.”
Doctors tend to look at bodily decline through the prism of so-called diseases of aging, our increasing susceptibility over time to killers like cancer and heart disease. But in the 1950s, research biologists began to view aging itself as the disease. When free radicals scavenge electrons from their neighbors, they set in motion some ugly chain reactions. Cholesterol molecules become oxidized and begin to interact with the artery walls to form atherosclerosis-causing plaque, for instance, or the DNA in the cell nucleus suffers mutations, laying the groundwork for cancer. Later refinements of this theory emphasize the role of the mitochondria, the cellular power plants that help convert glucose into energy. As the mitochondria age, they spew out increasing amounts of the free radicals that hamper energy production and damage the entire cell, accelerating our all-systems decline.
Among cell biologists, these mechanisms remain to this day the most accepted ways of explaining what’s happening to that face reflecting back at us in our bathroom mirror. But telomere science has opened up the possibility of drilling even deeper into the molecular bedrock of aging. The fledgling field was energized in 1984, when biochemist Elizabeth Blackburn of the University of California at Berkeley and her then-grad student Carol Greider discovered the telomerase enzyme in a pond-scum protozoan, an achievement that won them a Nobel Prize. Since then, our picture of human telomeres and telomerase has sharpened considerably.
“A magic pill?” says Nobel Prize winner Elizabeth Blackburn. “I think we’ve been there about a million times before.”Telomeres are made of repeating sequences of six DNA bases—two thymine, one adenine, three guanine (TTAGGG)—that serve to “cap” chromosomes, preventing potentially cancerous breaks; the analogy usually trotted out is the plastic aglet that prevents a shoelace from fraying at the ends. Telomeres also assist cell division. Every time a cell splits, the ends of its chromosomes fail to get fully copied in the two new daughter cells, and a bit of telomeric DNA gets lost. No harm is done to the rest of the chromosome, but in cells that divide frequently, the telomeres shorten with each replication. Telomerase’s job is to synthesize new DNA to add to the shrinking telomeres, slowing down the decline.
Human life, it turns out, is a losing effort to hang on to our telomeres. At conception, telomeres have roughly 15,000 DNA base pairs. Because telomerase can’t keep up with rapid cell division in utero, they shrink to about 10,000 base pairs at birth. At that point, the telomerase gene is mostly turned off. Without the enzyme, we continue to lose telomeric DNA—once we’re out of our teens, usually at a rate of 50 base pairs a year. By the time some of our telomeres drop below about 5,000 base pairs, typically well into our “golden” years, our cells may have lost the ability to divide. They become senescent, bad at doing the work they were designed to do but good at doing things like releasing inflammatory chemicals that harm their neighbors. Or they may be targeted for cell death.
Andrews sounds almost giddy when he describes the “aha” moment 20 years ago when he first heard his soon-to-be boss at Geron, pioneering telomere biologist Calvin Harley, lecture about telomeres as a “mitotic clock,” in which the steady shortening of the telomeres serves as the tick-tock of the aging cell. “I was floored,” Andrews says. He found the lockstep precision suggested by the metaphor irresistible.
Cultured in the lab, cells can divide just 50 to 70 times before packing it in (this is known as the Hayflick Limit, after longevity-research eminence Leonard Hayflick, who discovered the phenomenon). The human body is significantly more complex than a petri dish, but some similar limit must be enforced there, Andrews says, to account for the fact that the maximum human life span is so tightly regulated, with the longest-lived humans making it to 100 and, to the best of our knowledge, nobody surviving past 125. If free-radical damage were really the primary driver of aging, he says, people’s rate of bodily decline would vary widely based on the amount of environmental damage they had absorbed, a major contributor to the free-radical load, and therefore so would their maximum life span. “But you can look at a person and have a 95 percent chance of guessing their age within five years,” he says. “There has to be some kind of internal clock ticking inside of us.”
Biologists continue to debate the extent to which aging at the cell level determines the aging of the whole organism. Most have argued that short or damaged telomeres aren’t as big a deal as Andrews, or even the more measured Harley, make them out to be. Tissues and organ systems that depend on cell division have a fair amount of reserve capacity, and the cells that seem to play the biggest role in our decline, neurons and heart-muscle cells, hardly replicate at all.
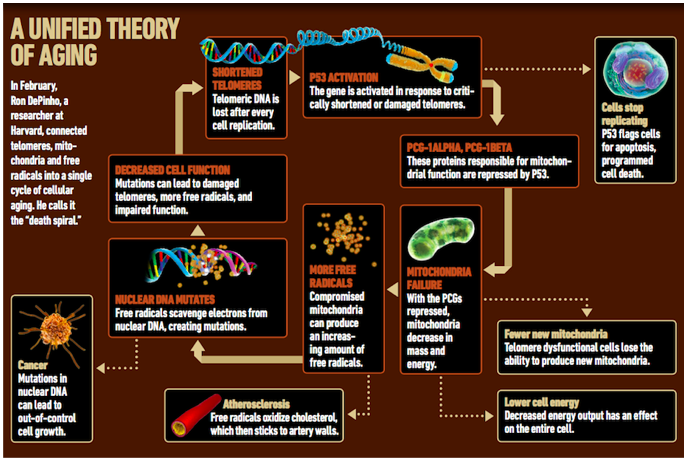
But over the past few years, the case for telomeres as a major player in aging, possibly even the prime mover, has grown stronger. Heart health, telomere biologists point out, depends heavily on the endothelial cells that line the blood vessels, and brain health on the glial and schwann cells that make the myelin that protects neurons, all of which are cell types that hear the ticking of the mitotic clock. And last year, Harvard University researcher Ron DePinho published two studies in the journal Nature that have reframed the debate about telomerase activation. DePinho created an ingenious model whereby he could turn telomerase off in a mouse and then restore it, simply by administering, or withholding, a synthetic estrogen drug. In the first study, the mice with turned-off telomerase exhibited signs and symptoms of decrepitude akin to what we might endure at the age of 80 or 90: wrinkled skin, sluggish intestines, shrunken brain. When telomerase production was turned back on, the tissues rejuvenated within a month.
“We treated these animals that were the equivalent of your grandmother,” DePinho says, “and they became like young adults.” He says he had expected to be able to stop or slow down the rate of aging. What he found was the proof-of-concept that living tissue could actually go back in time. (When Andrews talks about the possibility of running a seven-minute mile at the age of 130, he’s got the Harvard mice for backup.)
The second Nature paper was DePinho’s attempt at developing a unified theory of late-life aging, “the death spiral,” as he calls it, that can transform a spry, alert 80-year-old into a shell of herself at 90 or 100 even in the absence of diagnosable disease. His mice data suggest that the major aging processes—free-radical damage, mitochondrial dysfunction, and short or damaged telomeres—interrelate and that the telomeres can instigate decline, acting as the first domino that sets in motion the rest. If the telomeres can be preserved, the entire system may be granted at least a temporary reprieve.
DePinho says he envisions more animal-model research leading to human clinical trials leading—years or, more likely, decades down the road—to FDA-approved drugs. The high-speed, low-rent workaround of a telomerase-activating supplement beyond the reach of the FDA doesn’t please him. “Even if you did get telomerase activity,” he says, “you sure as hell would want to know where and when to turn it on. Telomerase can be deleterious as well.” Elizabeth Blackburn, now at the University of California at San Francisco, has reservations about a good-for-what-ails-you supplement. “A magic pill?” she says. “I think we’ve been there about a million times before in human history.”
Sierra Sciences operates out of a small, dun-colored office park near downtown Reno. From the outside, it could be mistaken for a Sun Belt Staples, but inside are touches that speak to Andrews’s specific history and sense of mission. He walks me into a conference room decorated with plaques commemorating U.S. patents issued, and a whiteboard with an “Aging Sucks” bumper sticker plastered on it. “Dad sent that,” Andrews says, identifying the handiwork of Ralph Andrews, a retired Los Angeles game-show producer (his biggest hit was You Don’t Say!, which ruled the daytime airwaves in the 1960s). For reasons Andrews can’t adequately explain, his father, still hale at 84, has always been dead set against aging, and once suggested to his preteen son that he might want to take a shot at solving the problem. “My dad probably told me to do a lot of things, but this just struck a chord,” he says. “I never thought aging was inevitable. I just thought nobody had figured it out yet.”
In the late ’90s, Andrews came to feel that Geron had lost the true telomerase-activating religion, having redirected most of its resources into stem-cell therapies. He left Geron, crossed the Sierras, and in 1999 gathered around him in the Nevada desert a small circle of researchers who believed almost as ardently as he that it might be possible to engineer a “small molecule” drug that would flip the telomerase gene’s “on” switch inside a living human body. Since then, the company has gone through two distinct phases, pre-crash and post-crash. In the first era, two especially beneficent investors unquestioningly underwrote his efforts to crack the telomerase code. (Start-ups working on an actual product in development attract venture capitalists. More-speculative ventures like Sierra Sciences typically draw individual “angels”—in the anti-aging field, often older, wealthy men willing to risk losing money in the hopes that somebody will come up with a way to extend their fruitful lives.)
During this first phase, Andrews and his team deployed an elegant recombinant DNA approach, arguably better suited to an academic lab than a start-up that needed marketable results. They would painstakingly alter one or two DNA bases out of the thousands that make up the telomerase gene, cycling through thousands of slight variations in an effort to find one that the regulatory molecule that normally keeps the gene turned off, the “repressor,” would no longer recognize. This would reveal the molecular identity of the repressor, and the team could then create a drug to neutralize it—repressing the repressor and switching the telomerase gene back on.
By 2006, after seven years of effort and one excruciatingly close miss (they found “a” repressor but apparently not “the” repressor), Andrews finally shifted strategies. If developing a telomerase-activating drug with recombinant-DNA methods was a bit like trying to find a needle in the haystack by analyzing the haystack molecule by molecule, the new approach was brute force: Grab a pitchfork and start digging. The company bought libraries of several hundred thousand chemical compounds and tested each one to see if it would activate telomerase in cultured human cells.
The cells Andrews chose were fibroblasts, which are found in skin and connective tissue and which are relatively cheap and easy to culture. They also have little ability to express telomerase in a lab setting. When Andrews first started the company, he ran into skepticism from some of his high-profile scientific advisers, who doubted his overall strategy of trying to turn on telomerase. “They were even laughing at it,” he says. Now at this later stage of the game, a few of his paid consultants questioned his decision to use fibroblasts. “Bill is the most persistent guy I’ve ever met,” says Bryant Villepointeau, a Geron alum and a former Sierra Sciences consultant. “Sometimes if he’s committed to something, he will go beyond the point where it’s wise.”
But Andrews had his reasons—the fibroblasts behave themselves in the lab and don’t change into other cell types, unlike stem cells, which can be moving targets. And after a year and a half of testing for telomerase activation, running compound after compound through a screening assay, he finally caught a break. On the 57,684th run, the team got a chemical hit. C0057684 was too toxic to be easily transformed into a drug prospect, but it gave the company a positive control. In other words, they could use it to tune their detection tests to recognize fainter and fainter levels of telomerase activation, which is essential when you’re working with stodgy, underperforming fibroblasts.
By then, however, the market crash of 2008 had clipped the wings of the company’s two angels, radically altering Andrews’s job description. Rather than spending his days and nights in the lab, he became a telomerase-activation evangelist, crisscrossing the country in search of funding. “Where’s Bill?” became a regular link on the company’s website. His doleful SOS bounced around the life-extension blogosphere: “The bottom line is that Sierra Sciences needs $200,000 per month as soon as possible.”
The worst part for Andrews was leaving the day-to-day responsibilities of the lab and retreating to his office, where he works the phones and e-mail trying to pilot the company out of financial peril. The long hours and personal austerity required by the new mission are by now second nature and, this afternoon, become grist for an enthusiastic show-and-tell. The office fridge: “For breakfast, I have a protein shake, and every two weeks I go to Trader Joe’s or Whole Foods and buy a whole bunch of frozen foods that I heat up for dinners.” The low-slung chest of drawers with the cushion on top where he spends many of his nights, cutting down on the commute in from his ranch 25 miles outside of town: “My legs overhang the edge, but that’s OK. If I bend my knees, my legs are on the cushion.” (The last bed I saw with such awkward dimensions belonged to Father Junipero Serra, the 18th-century founder of the California Franciscan missions—his attempt to mortify the flesh presented a resonant contrast to Andrews’s efforts to make it something closer to immortal.)
“Unequivocally, he’s paid a price with his scientific peers,” Federico Gaeta says. “How big, I don’t know. But Bill’s not going to break.”For all the monastic devotion he brings to the cause, Andrews is a pure gene jock. It’s a sign of our nutraceutical-besotted times that such a scientist has made a marriage of convenience with a supplement industry often equated with hippie herb lovers and cynical marketers looking to exploit the next pseudoscience fad. Gone are the bulk shipments of synthetic chemicals to be assayed, replaced by a small weekly delivery of ingredients derived mostly from traditional Chinese and Indian medicinal herbs that John W. Anderson prepares in his five-man Arizona lab. To Andrews’s surprise (and considerable relief), at least three of these compounds have tested positive for telomerase activation in the lab, even though many of the source materials are readily available in health-food stores. Have longtime devotees of traditional Chinese and Indian medicinal herbs been activating their telomerase without knowing it? Anderson, a self-described nutraceutical research scientist and medicine hunter, demurs, saying only that his nonchemical extraction and refining process concentrates and enhances any healing properties they may have previously exhibited. As Jon Cornell, Andrews’s administrative lieutenant at Sierra Sciences, says, if herbs and roots naturally had the level of telomerase-inducing activity that Andrews and his team are really looking for, “we’d probably already have immortal people.”
 Andrews leads me through a succession of compact lab rooms, each of which contains more equipment than people to run it. (Since 2008, he has cut the number of staff scientists from 34 to eight.) The center of the complex is a single cramped room where a couple of cell biologists and lab techs tend to plastic flasks holding millions of human fibroblast cells. The cells will be transferred to tiny plastic vials, frozen in liquid nitrogen, and then, when their number is called, thawed and bathed for 24 hours in one of Anderson’s natural ingredients. Then they’re whisked across the hall, where another small group of scientists and techs run a production line that sends plates of the treated cells through a LightCycler analyzer, which amplifies what’s going on at the molecular level using PCR (polymerase chain reaction, better known as the perp-catching technology on CSI). Telomerase is made up of two components—the RNA, which serves as a template to be used by the second part, a catalytic protein that synthesizes the DNA added back to telomeres. The LightCycler scans for RNA activity suggestive of telomerase expression. Promising compounds are then run through a slower, by-hand assay to look for hard evidence of the protein at work. “It’s cherry picking,” Andrews says. “The machine selects the reddest cherries.”
Andrews leads me through a succession of compact lab rooms, each of which contains more equipment than people to run it. (Since 2008, he has cut the number of staff scientists from 34 to eight.) The center of the complex is a single cramped room where a couple of cell biologists and lab techs tend to plastic flasks holding millions of human fibroblast cells. The cells will be transferred to tiny plastic vials, frozen in liquid nitrogen, and then, when their number is called, thawed and bathed for 24 hours in one of Anderson’s natural ingredients. Then they’re whisked across the hall, where another small group of scientists and techs run a production line that sends plates of the treated cells through a LightCycler analyzer, which amplifies what’s going on at the molecular level using PCR (polymerase chain reaction, better known as the perp-catching technology on CSI). Telomerase is made up of two components—the RNA, which serves as a template to be used by the second part, a catalytic protein that synthesizes the DNA added back to telomeres. The LightCycler scans for RNA activity suggestive of telomerase expression. Promising compounds are then run through a slower, by-hand assay to look for hard evidence of the protein at work. “It’s cherry picking,” Andrews says. “The machine selects the reddest cherries.”
The analogy sounds so delightful that it’s jarring to remember that the measuring rod, the “standard control” the lab uses to evaluate telomerase activity in test compounds, is cancer—specifically the HeLa cancer cells that were the first cell line to achieve immortality. Back when Andrews was working with the more potent synthetic chemicals that he says were, in theory, capable of putting the brakes on aging, his team was able to get one compound up to a 16. That would be 16 percent of the telomerase required to make the HeLa cells live forever. “What we really want to do is to get it to 100 percent and above,” he says.
Telomerase, as Blackburn once noted, is a Dr. Jekyll and Mr. Hyde proposition. Though it will not cause a cell to turn cancerous by itself, telomerase in its uncivilized Mr. Hyde mode does fuel the unregulated growth of most cancers. By activating the enzyme, Calvin Harley says, “there is a risk, a small probability, that it could cause a premalignant cell to divide enough times to become malignant.” But both Harley and Andrews say they believe that any increased cancer risk is outweighed by the potential rewards. Telomerase can also be a benign Dr. Jekyll that protects against the chromosomal breakage and re-fusion that can lead to cancer, and it can help drive the proliferation of immune-system cells whose job it is to fight cancer.
A study in the July 7, 2010, Journal of the American Medical Association highlighted the correlation between cancer and short telomeres: People with shorter-than-average telomeres had three times the risk of developing cancer and 11 times the risk of dying from it. Andrews is not shy about talking with cancer patients—seemingly the group most vulnerable to the Mr. Hyde risks of runaway telomerase—about the potential health advantages of telomerase activation. “I’m always careful to qualify that I’m not an M.D., I’m not able to provide medical advice,” he says. “I do say that if I had cancer, I’d be taking as much telomerase activator as I could get my hands on.”
As it happens, he already is. In 2002, a New York City entrepreneur and former appliance manufacturer, Noel Thomas Patton, licensed the rights to Geron’s research on a telomerase-activating compound found in the Chinese medicinal herb astragalus, for supplement use only. (Geron is finalizing a plan to send an astragalus-based telomerase-activating drug candidate through clinical trials.) Three years ago, Patton’s TA Sciences test-launched its TA-65 supplement with 100 clients, each willing to pay $25,000 a year to be anti-aging guinea pigs. Paying patient number one: Bill Andrews.
TA Sciences has this year ramped up production and dropped the stratospheric price tag, although so far the most impressive effects remain anecdotal—more energy, greater mental clarity, a sexual boost, even improved vision. Andrews says his ultramarathon times dropped when he started taking TA-65. An observational study co-authored by Harley, who helped discover the original molecule at Geron, found improvements in the immune system of those first 100 clients. Andrews was hoping for a more pronounced effect. As he describes what it was like to take that first dose of the supplement in 2008, I can hear the voice of a kid who hasn’t entirely grown up, anti-aging as a never-ending Hardy Boys adventure: “I remember Noel and I sitting having dinner, and we were wondering, What are we going to look like two weeks from now? We talked on the phone practically every day, and we were both disappointed that we didn’t look any younger right away.”
Andrews’s tendency to let his enthusiasms take him out on a limb, especially when he’s trying to attract investors, makes him a polarizing figure in the research community. To some academics, his standard pitch-cum-sound-bite, “We age because our telomeres shorten,” is a crude oversimplification. Even Andrews seems to suspect that Sierra Sciences’s company motto, “Cure Aging or Die Trying,” isn’t winning him many friends among people who possess advanced biology degrees. “Some people like it and other people say it’s embarrassing,” he says. “So I don’t know what to do.”
“I can’t be happy unless I’m working on this,” Andrews says. “The mission won’t die unless I die.”I later ask Federico Gaeta, Geron’s former head of chemistry and a current Sierra Sciences consultant, whether Andrews’s reputation has suffered for his damn-the-nuance pursuit of longevity. “Unequivocally, he’s paid a price with his scientific peers,” he says. “How big a price, I don’t know, but there is an excellent chance that he will ultimately be vindicated.” Now, Gaeta says, “he’s in a position where he has to show that he’s done something.” The years of angels with blank checks are over, and the pressure to produce—and to raise the money to buy the time to produce—is tremendous. “He’s not going to break,” Gaeta says. “I know that about him. Bill’s not going to break.”
By 5 P.M., midwinter darkness is beginning to fall, and the skeleton crew at Sierra Sciences is mostly gone, though Andrews is looking at another long night that will probably end on his makeshift bed. The last employee to leave is Randy Lee, the IT guy, an old Southern California prep-school buddy of Andrews’s. He’s been hanging around because he has some bad news to deliver. Lee has the unenviable job of reconfiguring the lab’s now inadequate computer system. Today he lost a cache of valuable data when the system crashed. When he delivers the news, Andrews visibly compresses, as if another 10 pounds has been added to the weight already on his shoulders. Then he collects himself. “I told people we’re either going to never move forward with our system or we’re going to take the chance of losing things,” he says. “Well, try to get a good night’s sleep. I’m sorry for your sake that it happened.”
After Lee heads for home, I ask Andrews to consider a hypothetical. If I wrote him a check for $10 million, would that be enough to send him back to the lab to find that home-run telomerase-activating chemical? “No,” he says, “but that would increase our chances of getting a really good natural product that nobody could compete with. To do the pharmaceutical, we’d need $30 million.” I toss out a flip rejoinder—“Sorry, Bill, I can only do the $10 million”—and Andrews freezes for a half-second, then slumps back in his chair. “I’ve got business plans that have all that budgeted,” he says. “What the money would be used for.”
I ask Andrews what the worst-case scenario would be for Sierra Sciences. “The worst-case scenario,” he says, “is that we put out a telomerase activator and everybody who takes it dies right away.”
“No,” I clarify, “the worst-case financial scenario?”
Andrews, his voice phlegmy with fatigue, tries again. “The company folds. I find another job, but I still work on trying to find more investors to resurrect it. I can’t be happy unless I’m working on this. The mission won’t die unless I die.”
Me: This was the same article I had read almost 2 years ago and I honestly don’t know how Sierra Sciences is coming along. I know that Bill Andrews is still on his mission to succeed in his mission to cure agin.
The main thing Andrews has been trying to do is to find a compound that can activate the telomerase in our body to a high enough level to slow down the shortening of telomeres.