Meclozine is available without a prescription: Meclizine Chewable Tablets – 25mg – Model 85207 – Btl of 100 . Open growth plates only though but it looks like it could be very effective as it is similar to CNP which has a pretty big impact on height.
. Open growth plates only though but it looks like it could be very effective as it is similar to CNP which has a pretty big impact on height.
Even if you don’t understand anything below. Please spread the word about this study. It looks like a quite promising OTC height increase supplement.
Unfortunately, there have been no studies on Meclizine and human height and as shown by the study below there is cell toxicity to Meclizine. Since Meclizine is a well established supplement, anyone who is currently undergoing longitudinal growth and wants to grow taller should take Meclizine at dosages recommended on the bottle and following all other directions about directed use.
Meclozine Facilitates Proliferation and Differentiation of Chondrocytes by Attenuating Abnormally Activated FGFR3 Signaling in Achondroplasia.
“Achondroplasia (ACH) is one of the most common skeletal dysplasias with short stature caused by gain-of-function mutations in FGFR3 encoding the fibroblast growth factor receptor 3. We used the drug repositioning strategy to identify an FDA-approved drug that suppresses abnormally activated FGFR3 signaling in ACH. We found that meclozine, an anti-histamine drug that has long been used for motion sickness, facilitates chondrocyte proliferation and mitigates loss of extracellular matrix in FGF2-treated rat chondrosarcoma (RCS) cells. Meclozine also ameliorated abnormally suppressed proliferation of human chondrosarcoma (HCS-2/8) cells that were infected with lentivirus expressing constitutively active mutants of FGFR3-K650E causing thanatophoric dysplasia, FGFR3-K650M causing SADDAN, and FGFR3-G380R causing ACH. Similarly, meclozine alleviated abnormally suppressed differentiation of ATDC5 chondrogenic cells expressing FGFR3-K650E and -G380R in micromass culture. We also confirmed that meclozine alleviates FGF2-mediated longitudinal growth inhibition of embryonic tibia in bone explant culture. Interestingly, meclozine enhanced growth of embryonic tibia in explant culture even in the absence of FGF2 treatment!!!!!!!. Analyses of intracellular FGFR3 signaling disclosed that meclozine downregulates phosphorylation of ERK but not of MEK in FGF2-treated RCS cells. Similarly, meclozine enhanced proliferation of RCS cells expressing constitutively active mutants of MEK and RAF but not of ERK, which suggests that meclozine downregulates the FGFR3 signaling by possibly attenuating ERK phosphorylation{Since everything . We used the C-natriuretic peptide (CNP) as a potent inhibitor of the FGFR3 signaling throughout our experiments, and found that meclozine was as efficient as CNP in attenuating the abnormal FGFR3 signaling!!!!!!!!{And CNP is a huge height increase disease}. ”
Loss of function of FGFR3 leads to tall stature and Meclozine decreases FGFR3 function even in normal cells.
“CNP has a short half-life and continuous intravenous infusion is required for in vivo experiments. The CNP analog with an extended half-life, BMN 111, has recently been developed and significant recovery of bone growth was demonstrated in ACH mice by subcutaneous administration of BMN 111″
” 0, 1, 2, 5, 10, and 20 µM of meclozine exhibited dose-dependent increases in RCS[Rat Chondrosarcoma cells] proliferation. We did not observe dose-dependency at 50 µM, which was likely due to cell toxicity. We also confirmed that 10 and 20 µM of meclozine increased the number of RCS cells”
“treating RCS cells with FGF2 for four hours induced expressions of matrix metalloproteinase 10 (Mmp10), Mmp13, and a disintegrin-like and metalloproteinase with thrombospondin type 1 motif 1 (Adamts1) transcripts{LSJL upregulates Admts1 and MMP13 but still increases height, LSJL in conjunction with Meclozine could increase height more}. We found that meclozine and CNP significantly suppressed expressions of these matrix metalloproteinases. We also quantified expressions of Col2a1 and Acan transcripts, but FGF2 treatment for 72 hours did not reduce the expression levels of these genes in RCS cells”
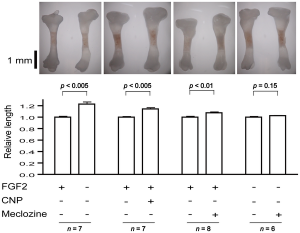 The rightmost figure(the one with n=6) is the Meclozine solo group. Eyeballing it, it looks like it could be about 5% increase in longitudinal growth. Note that a 5% increase on 5’9″ is 6’0″.
The rightmost figure(the one with n=6) is the Meclozine solo group. Eyeballing it, it looks like it could be about 5% increase in longitudinal growth. Note that a 5% increase on 5’9″ is 6’0″.
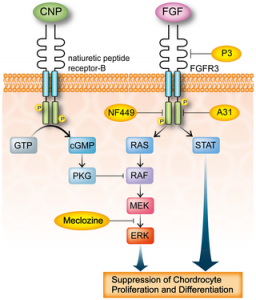 FGFR3 signaling in chondrocytes. LSJL does increase ERK phosphorylation. Maybe that is a side effect of the boost of LSJL on longitudinal growth and not a cause of longitudinal growth. And that both Meclozine and LSJL would have additive effects.
FGFR3 signaling in chondrocytes. LSJL does increase ERK phosphorylation. Maybe that is a side effect of the boost of LSJL on longitudinal growth and not a cause of longitudinal growth. And that both Meclozine and LSJL would have additive effects.
” a synthetic compound A31 is an inhibitor of the FGFR3 tyrosine kinase by in silico analysis. They demonstrated that A31 suppresses constitutive phosphorylation of FGFR3 and restores the size of embryonic femurs of Fgfr3Y367C/+ mice in organ culture. In addition, A31 potentiates chondrocyte differentiation in the Fgfr3Y367C/+ growth plate. P3 has a high and specific binding affinity for the extracellular domain of FGFR3. They showed that P3 promotes proliferation and chondrogenic differentiation of cultured ATDC5 cells, alleviates the bone growth retardation in bone rudiments from TD mice (Fgfr3Neo-K644E/+ mice), and finally reversed the neonatal lethality of TD mice”
“These novel FGFR3 tyrosine kinase inhibitors, however, may inhibit tyrosine kinases other than FGFR3 and may exert unexpected toxic effects in humans. Meclozine may also inhibit unpredicted tyrosine kinase pathways, but we can predict that there will be no overt adverse effect, because meclozine has been safely used for more than 50 years.”
Here’s a study that will provide some insight into FGFR3 and why it inhibits growth in some cases but not in others:
The paradox of FGFR3 signaling in skeletal dysplasia: why chondrocytes growth arrest while other cells over proliferate.
“Somatic mutations in receptor tyrosine kinase FGFR3 cause excessive cell proliferation, leading to cancer or skin overgrowth. Remarkably, the same mutations inhibit chondrocyte proliferation and differentiation in developing bones, resulting in skeletal dysplasias, such as hypochondroplasia, achondroplasia, SADDAN and thanatophoric dysplasia{So maybe the longitudinal growth induced by LSJL is not produced by chondrocytes but rather by the stem cells which would be promising evidence for post LSJL-inducable height growth}. A similar phenotype is observed in Noonan syndrome, Leopard syndrome, hereditary gingival fibromatosis, neurofibromatosis type 1, Costello syndrome, Legius syndrome and cardiofaciocutaneous syndrome. Collectively termed RASopathies, the latter syndromes are caused by germline mutations in components of the RAS/ERK MAP kinase signaling pathway. This article considers the evidence suggesting that FGFR3 activation in chondrocytes mimics the activation of major oncogenes signaling via the ERK pathway. Subsequent inhibition of chondrocyte proliferation in FGFR3-related skeletal dysplasias and RASopathies is proposed to result from activation of defense mechanisms that originally evolved to safeguard mammalian organisms against cancer.”
“FGFR3 [inhibits] chondrocyte proliferation. FGFR3/ERK signaling triggers disintegration of the cyclin D3-cdk6 complex in the G1 phase of the cell cycle, followed by increased association of p21WAF1 and p27Kip1 cell cycle inhibitors (CKI) with cyclin-cdk2 and cyclin-cdk4 complexes, leading to inhibition of their kinase activities. Upon FGFR3 activation, CKIs accumulate at the protein level, due to the interaction with transcriptionally induced cyclin D1. cyclin D1 upregulation also mediates the pro-mitogenic effects of FGFR/ERK signaling. Duration, magnitude and timing of cyclin D1 and p21WAF1 induction in the G1 phase of a cell cycle determines the nature of the response to an ERK signal. A strong but transient ERK activation in the early G1 induces intermittent p21WAF1 accumulation and stable cyclin D1 expression, leading to cell proliferation. In contrast, robust and persistent ERK activation leads to stable p21WAF1 accumulation and growth inhibition despite the concomitant induction of cyclin D1.
In chondrocytes, unlike most other cell types, FGFR3 activation elicits highly prolonged ERK activation lasting for up to 24 h. This phenotype is likely to stem from the maintenance of ERK pathway activation within the protein complexes interacting directly with FGFR3. ”
So FGFR3 inhibits growth in chondrocytes but not other cell types is because FGFR3 activates ERK for too long which leads to growth inhibition due to excess levels of p21WAF1.
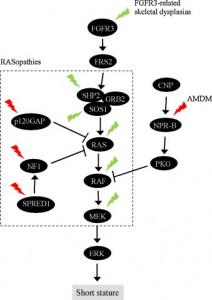 “In chondrocytes, the premature senescence caused by FGFR3 activation does not involve the p53 pathway but appears CKI-dependent as induction of several CKIs (p21WAF1, p27Kip1, p16INK4a, p18INK4c, and p19INK4d) accompanies FGFR3-mediated inhibition of chondrocyte proliferation in vitro and in vivo”
“In chondrocytes, the premature senescence caused by FGFR3 activation does not involve the p53 pathway but appears CKI-dependent as induction of several CKIs (p21WAF1, p27Kip1, p16INK4a, p18INK4c, and p19INK4d) accompanies FGFR3-mediated inhibition of chondrocyte proliferation in vitro and in vivo”
NEW:
Meclozine Promotes Longitudinal Skeletal Growth in Transgenic Mice with Achondroplasia Carrying a Gain-of-Function Mutation in the FGFR3 Gene.
“Achondroplasia (ACH) is one of the most common skeletal dysplasias causing short stature owing to a gain-of-function mutation in the FGFR3 gene, which encodes the fibroblast growth factor receptor 3. We found that meclozine, an over-the-counter drug for motion sickness, inhibited elevated FGFR3 signaling in chondrocytic cells. To examine the feasibility of meclozine administration in clinical settings, we investigated the effects of meclozine on ACH model mice carrying the heterozygous Fgfr3ach transgene. We quantified the effect of meclozine in bone explant cultures employing limb rudiments isolated from developing embryonic tibiae from Fgfr3ach mice. We found that meclozine significantly increased the full-length and cartilaginous primordia of embryonic tibiae isolated from Fgfr3ach mice. We next analyzed the skeletal phenotypes of growing Fgfr3ach mice and wild-type mice with or without meclozine treatment. In Fgfr3ach mice, meclozine significantly increased the body length after two weeks of administration. At skeletal maturity, the bone lengths, including the cranium, radius, ulna, femur, tibia, and vertebrae were significantly longer in meclozine-treated Fgfr3ach mice than in untreated Fgfr3ach mice. Interestingly, meclozine also increased bone growth in wild-type mice. The plasma concentration of meclozine during treatment was within the range that has been used in clinical settings for motion sickness. Increased longitudinal bone growth in Fgfr3ach mice by oral administration of meclozine in a growth period indicates potential clinical feasibility of meclozine for the improvement of short stature in ACH.”
“A CNP analog with an extended half-life, BMN-111, has recently been developed, and significant bone growth recovery was demonstrated in amouse model of ACH by subcutaneous administration of BMN-111”
“Meclozine was administrated to 2-week-old wild-type mice for 3 weeks. As wild-type mice were weaned at 2 weeks after birth, we started meclozine treatment 1 week earlier than that for Fgfr3ach mice. The body length of meclozinetreated mice was significantly longer than that of untreated mice after 1 week”
“the plasma concentrations of meclozine used in the current study (0.2 or 0.4 g of meclozine per kilogram food).”
“Meclozine, an OTC H1 inhibitor, has been safely used for motion sickness for more than 50 years, and its optimal dose and adverse effects have already been established”
“in patients with ACH [given treatment with meclozine], the patients could be expected to increase 6.7 to 7.1 cm in height, based on the average height of adults with ACH.”
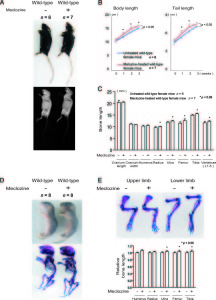 <-Treatment of mice with meclozine without FGFR3 deficiencies. The meclozone treated mice are noticeably taller and lengthier.
<-Treatment of mice with meclozine without FGFR3 deficiencies. The meclozone treated mice are noticeably taller and lengthier.
“A-C, Wild-type mice were treated with meclozine 2 weeks after birth for 3 weeks. A, Visual images and soft X-ray images of wild-type female mice with or without meclozine. Meclozine-treated mice were larger than the untreated mice. B, Body and tail lengths of meclozinetreated
wild-type female mice were significantly longer than those of untreated wild-type female mice. Statistical significance analyzed by two-way ANOVA is shown on the right side of each graph. *P � .05 by Fisher’s LSD test for each pair. C, The lengths of the radius, ulna, femur, tibia, and vertebrae on the soft X-ray films were significantly increased by meclozine treatment by unpaired t test. D and E, Pregnant mice were treated with meclozine from embryonic day 14. D, Visual images and skeletons stained with Alizarin red and Alcian blue of wild-type mice at postnatal day 5, with or without meclozine. Meclozine-treated offspring were larger than untreated offspring. E, The lengths of the ulna, femur, and tibia measured using stained skeletons were significantly increased after meclozine treatment, as assessed by unpaired t test.”
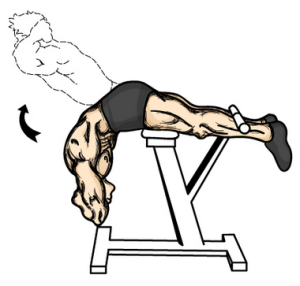 Here is something that I recently found which will help a large percentage of people which will let them gain about half a centimeter in increase height quite quickly. Something which I have always believed is that getting exercise which can remove the load on our backs and vertebrate bone will lead to some height increases.
Here is something that I recently found which will help a large percentage of people which will let them gain about half a centimeter in increase height quite quickly. Something which I have always believed is that getting exercise which can remove the load on our backs and vertebrate bone will lead to some height increases.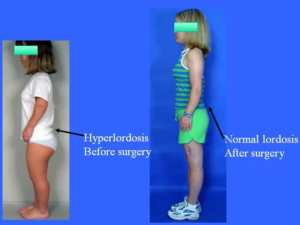 Refer to the Youtube Video “Achondroplasia A Guide For Parents By Dr Dror Paley.mp4”. Notice how the lower back area is curved towards the posterior direction.
Refer to the Youtube Video “Achondroplasia A Guide For Parents By Dr Dror Paley.mp4”. Notice how the lower back area is curved towards the posterior direction.



