One of the issues behind neo-growth plate formation in adults is that adult MSCs are different from embryonic stem cells. So can adult MSCs form new growth plates despite being more heterogeneous than embryonic stem cells and having less replicative potential? Are the stem cells that become the epiphyseal growth plate special and have effects that cannot be replicated by adult mesenchymal stem cells?
Common Skeletal Growth Retardation Disorders Resulting from Abnormalities within the Mesenchymal Stem Cells Reservoirs in the Epiphyseal Organs Pertaining to the Long Bones
“the key role of the cartilage-bone regions is their responsibility to replenish the physis with committed chondrocytes, during the developmental, maturation and puberty periods.”
“The concentration gradients of substances such as decreased nutrients, decreased oxygen and decreased specific agents, such as NADNicotinamide-adenine-dinucleotide and poly ADP (Adenosine diphophate) ribose, most probably resulting due to the devascularization [affect morphogenic development].”
“The common metabolic features of a cartilaginous tissue are as follows: A tissue poor in cell numbers, a lack of vascularity, lymph and neurits. Also, slow, glycolytic metabolism, low oxygen tension, flagmatish nature and behaviour expressed in cell proliferation, with special capabilities and routes for the synthesis and secretion of extracellular substances. The spatial three-dimensional structure of the tissue is responsible for the tissue function{so maybe another structure could replicate growth plate function?}, rather than the cells themselves. They possess poor capability for wound healing and regeneration; cells present their final differentiation and maturation stages”
“The nutrition of the cartilaginous tissues is mainly by diffusion of small molecular weight
substances as well as limited diffusion of charged molecules. The transport barrier consists of the negative sulfate and carboxylic groups. The collagens and the proteoglycans carry the main mechanical and biomechanical responsibilities, the hydratation-imbibition of the proteoglycans
contribute to the resistance to compressive strains and their distribution, while the collagen fibers are responsible for the tensile strength.”
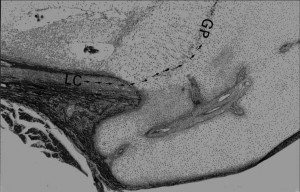
“cells arrive from a peripheral site of the physis – the so-called La Croix’s ring and Ranvier’s groove, at the interface of the epiphysis – metaphysis –periosteum – perichondrium, as the sanctum of the developmental activities of the precartilaginous stem cells. At these centers of cell populations and along the routes of their migration towards the germ cell zone of the
physis, chemoattractants, receptors and ligands operate in concert, inducing specific recruitment mechanisms”
“Migration assays of cells in vitro by chemoattractants and in vivo migration testing by
an operational approach, installing a flap of tissue as a barrier on the way of cell migrating route, events that lead to a change in their migration direction, almost by 90°, in pendicular to the physis”
“The epiphyses of the long bones behave as organs with specific functions during the developmental processes, responsible for normal skeletal growth, while pathological growth
retardation maladies are expressed as mutations (EXT1&2 and FGFR-3) within the very same cell populations. Among the pathologies the current review will be focused on two of the main
disorders: Osteochondromas, Hereditary Multiple Exostoses (HME) and solitary exostosis, and
Achondroplastic dwarfism; both ailments are initiated and originally expressed in the
mesenchymal stem cell reservoir populations of the epiphyses”
“At the margins of the periphery of the growth plate, at the metaphysis, on the border between
the epiphyses and diaphysis, exist a population of unique cells with the morphology of fibroblasts, present at the early stages of life, populating the structures of the so-called La Croix’s ring and Ranvier’s groove. These cell centers, also named metaphyseal perichondrium, are believed to be the storage sites of the mesenchymal stem cells,
prone to undergo several differentiation steps within the chondrogenic anlage direction. Starting with prechondroblasts and heading towards mature chondrocytes, simultaneously with the migration of the cells from the physeal periphery to the top of the growth plate columns, they replenish the physis with new differentiated – mature chondrocytes.{We have to mimic these events in order to grow taller} These events take place and last all along the developmental stages and ended post puberty, with the closure and the elimination of the physis. The above described events relate to the healthy state of matters. However under
disturbed, pathological circumstances, the cellular differentiative fate and the cellular migration are deviated, leading to extreme clinical manifestations.
a) Formation of bone protuberances perpendicular to the growth plate, the so-called exostoses
as a result of mutations in the EXT tumor suppressing genes coding for glycosyltransferases, responsible for the synthesis of heparan sulfate molecules. Heparan sulfate molecules in the pericellular sites are known to serve as activators of FGF receptors. These receptors in turn control the metabolic activity of the cells. In states of shortage, or complete lack of heparan sulfate molecules, the direction of cell migration seems to be altered from upward, along the
periphery of the physis, to perpendicular to the growth plate. The final outcome is the
formation of bone protuberances and clinical difficulties including pain, short stature, as
well as a potential to undergo malignant transformation to chondro and osteosarcoma,
since the healthy EXT genes are considered as malignant tumor suppressing genes. Further
details on HME-osteochondromas clinical entities are outlined and differentiated from
the solitary osteochondroma in the next paragraph on neoplastic disorders.
b) Failure of proper function of FGFR3 (fibroblast growth factor receptor 3) (FGF receptor3) with a negative control in response to its ligand FGF-9, expressed as a deviation of the migration of cells. Instead of migrating to the top of the physis columns and elongating the bone height, cells remain in their original vicinity at the physis periphery and cause the typical widening of the epiphyseal head diameter”{how do we induce this migration?}
“In Achondroplasia there is widening of the long bone heads at the metaphyseal regions which is most probably due to the accumulation of cells that fail to migrate due to the mutation in
FGFR3. The responsible cells for both phenomena are the mesenchymal stem cells – the
prechondrocytes in the reservoirs, that fail to migrate along the proper route due to improper
signalling molecules.”
“Improper concentrations of HA, e.g. overproduction induced by Has 2 in the mesoderm, leads to severely malformed –shortened limbs, lacking one or more skeletal elements, with abnormal morphology and inappropriate positioning of the limb elements.
The sustained production of HA perturbs limb development, affecting precartilaginous condensations, chondrogenesis, growth patterning and cartilage differentiation, normal progression and chondrocyte maturation”
“Cell migration tests have been developed by our team in tissue culture plates. Human mesenchymal-precartilagonous cells were seeded to confluence on a half of the plates’ surface,
while an examined agent (chemoattractant) was placed – embedded in a gel of agarose within a
glass ring at the other pole of the plate. Cells from the confluent half migrated towards the other pole pending on the gradient of the treatment. Among the examined agents were TNF γ; IL-LTB-4; c- AMP; MCP-1 (monocytes chemoattractantprotein-1); IL-1; GM-CSF (granulocyte macrophage colony stimulating factor); IL-2; leptin. The last two agents (IL-2 and leptin) accelerated migration of the cultured cells towards the ring containing the chemoattractant.”
“The last two agents (IL-2 and leptin) accelerated migration of the cultured cells towards the ring containing the chemoattractant.”<-Leptin has been established as having a role in endochondral ossification before. IL-2 will have to be investigated later.
“Leptin affects the chondrogenic axis lineage, especially at the early phase of chondrogenic differentiation, chondrogenic proliferation and differentiation in the epiphyseal growth plate.”
“Leptin is believed to accelerate skeletal growth via the expression of IGF-I receptors.”
“Osteochondromas are usually classified as benign bone tumors, but they are not neoplastic in nature{not growing new tissue}.
They appear to result from aberrant epiphyseal development with displacement of physeal
cartilage through the perichondrial fibrous ring and subsequent growth at right angles to the long axis of the bone. The lesion is composed of a mature bony stalk that is covered by a cartilaginous cap”
“removal of the ring of La Croix by surgery causes a complete growth arrest of the limbs”<-Would installation of a new ring of La Croix result in a growth restoration?
“Exposure of the periost to TGF-β1 enhances periosteal chondrogenesis”
“Periosteal chondroma is a benign cartilage-forming lesion, located on the surface of the bone, under the periosteum. It is also referred to as juxtacortical chondroma. Priosteal osteosarcoma is a low- to intermediate grade bone-forming sarcoma with predominantly chondroblastic differentiation that develops on the surfaces of long bones in children”
“[These compounds have a migration effect on chondrocytes]: PDGF, IGF-I, TGF-beta, and Hepatocyte growth factor, BMP-2, IL-1 and 2, and calcitonin”
“adhesive molecules and integrins, HA and its cell surface receptors affect cell mobility and migration”
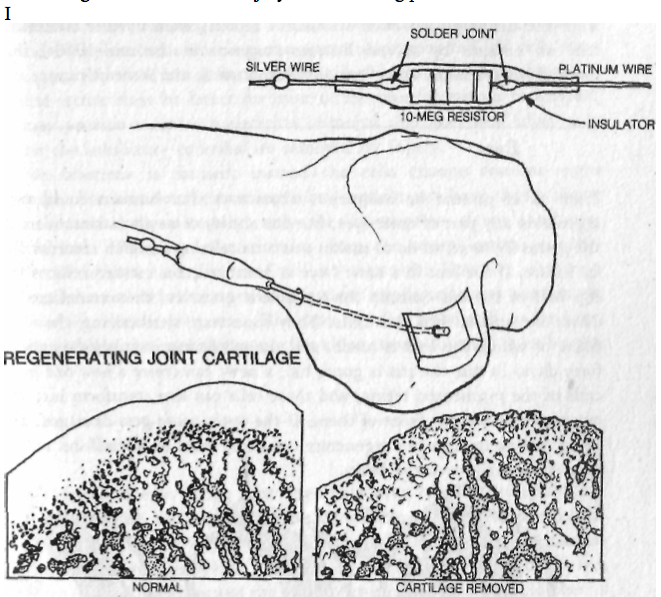
Image of Ring of La-Croix(LC):
Fibroblast growth factor receptor-3 as a marker for precartilaginous stem cells.(Full Study not available)
“The epiphyseal organ contains two kinds of cartilage, articular and growth plate. Both enlarge during the growth phase of life. However, mitosis is not apparent in these tissues. In the current study, a search to trace the reservoirs of stem cells needed for the growth of these cartilages is done. A disorder in which the stem cells responsible for bone growth are mutated is achondroplasia; the mutation resides in the fibroblast growth factor receptor-3. Epiphyses stained with antifibroblast growth factor 3 antibodies reveal clusters of positively stained cells residing in the perichondrial mesenchyme, known as the ring of La Croix. Removal of the ring of La Croix causes a drastic growth arrest in the limbs of rat neonates. Cell cultures derived of the ring of La Croix biopsy specimens show high rates of cell proliferation and cell migration in vitro, in contrast to articular or growth plate derived chondrocytes. These cells stain intensely by antifibroblast growth factor receptor-3 antibodies and antiproliferative cells nuclear antigen, in contrast with articular and epiphyseal chondrocytes. Transfection of cells from the ring La Croix by an adenovirus vector containing the gene encoding for Escherichia coli beta-galactosidase (lacZ), allows tracing of these cells in tissues. Local injections were performed either to the ring of La Croix or to the joint cavity in a guinea pig model. A characteristic distribution was seen after injection. The transfected cells migrated to areas of bone and cartilage formation in the subchondral bone plate and on either side of the growth plate. This labeling and distribution is maintained for as many as 3 months after injection. The cells from the ring of La Croix appear to be responsible for bone growth. Furthermore, perichondrial cells and other precartilaginous cells expressing fibroblast growth factor-3 have been shown to be good cells for implantation to correct defects of articular cartilage.”
CMF608-a novel mechanical strain-induced bone-specific protein expressed in early osteochondroprogenitor cells.
“Microarray gene expression analysis was utilized to identify genes upregulated in primary rat calvaria cultures in response to mechanical force. One of the identified genes designated CMF608 appeared to be novel. The corresponding full-length cDNA was cloned and characterized in more details. It encodes a putative 2597 amino acid protein containing N-terminal signal peptide, six leucine-rich repeats (LRRs), and 12 immunoglobulin-like repeats, 10 of which are clustered within the C-terminus. Expression of CMF608 is bone-specific and the main type of CMF608-positive cells is mesenchymal osteochondroprogenitors with fibroblast-like morphology. These cells reside in the perichondral fibrous ring of La Croix, periosteum, endosteum of normal bone as well as in the activated periosteum and early fibrous callus generated postfracture. Expression of CMF608 is notably absent from the regions of endochondral ossification. Mature bone cell types do not produce CMF608 with the exception of chondrocytes of the tangential layer of the articular cartilage, which are thought to be under constant mechanical loading. Ectopic expression of CMF608 in HEK293T cells shows that the protein is subjected to post-translational processing and its N-terminal approximately 90 kDa polypeptide can be found in the conditioned medium. Ectopic expression of either the full-length cDNA of CMF608 or of its N-terminal region in CMF608-negative ROS17/2.8 rat osteosarcoma cells results in transfected clones displaying increased proliferation rate and the characteristics of less-differentiated osteoblasts compared to the control cells. Our data indicate that CMF608 is a unique marker of early osteochondroprogenitor cells. We propose that it could be functionally involved in maintenance of the osteochondroprogenitor cells pool and its down-regulation precedes terminal differentiation.”
“Mechanical triggering of rat primary calvaria cells was achieved by stretching following irreversible deformation of the cell attachment surface of the culture dish. For the facilitated identification of de novo transcribed genes, nuclear RNA was extracted following 20 min of stretching.”
“genes transactivated following mechanical strain [include] tenascin C, collagen type XII{up in LSJL}, fibronectin, and connective tissue growth factor”
“genes like ADAMTS-1{up}, thrombospondin 1, endothelin-1{up in LSJL} (its activating enzyme was found upregulated), and desmoykin (AHNAK) [are] previously associated with osteoblastic differentiations.”
No other genes from Table 1 were found to be shared between this and LSJL.
“CMF608 expression was significantly increased in response to all the bone formation promoting stimuli applied, the maximal effect being caused by estrogen administration. In contrast, sciatic neurotomy led to down-regulation of CMF608 mRNA levels”
“In situ hybridization analysis revealed peculiar topography and cell specificity of CMF608 expression in the skeletal tissue. The hybridization signal delineated accumulations of fibroblast-like cells in several locations throughout the long bone: perichondral fibrous ring of LaCroix, periosteum, Volkmann’s canals and endosteum”
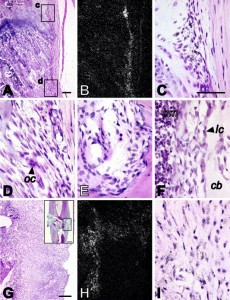
“n situ hybridization analysis of CMF608 expression in normal and fractured rat bones. Hybridization signal is evident as dark dots in the higher magnification brightfield images (C, D, E, F, I), or as white dots in the lower magnification darkfield images (B, H). Expression of CMF608 mRNA in normal rat tibia is shown in panels A–F. Expression of CMF608 in the healing fracture callus 1 week after the fracture is shown in panels G–I. Panels A and B are respectively low power brightfield and corresponding darkfield microphotographs of the metaphysis. Boxed areas, c and d, shown in panel A, are presented at higher magnification in panels C and D, respectively. Hybridization signal for CMF608 concentrates over fibroblast-like cells in the areas of perichondral fibrous ring (C) and metaphyseal periosteum (D). No hybridization signal is detected above progenitor cells in primary and secondary spongiosa. Panel E demonstrates expression of CMF608 in mesenchymal cells within Volkmann’s canal. Panel F shows expression of CMF608 in endosteal fibroblast-like cells in tibial diaphysis. No hybridization signal is detected above osteoblasts. Panels G and H are, respectively, low-power brightfield and corresponding darkfield microphotographs of the area of fracture callus, which is boxed in the inserted image appearing in the right upper corner of panel G. Hybridization signal concentrates over the fibrous part of the callus but not above the cartilaginous or woven bone areas. Panel I is a high power image of the fibrous part of the callus. Hybridization signal is associated with fibroblast-like cells. oc—osteoclast, bm—bone marrow, lc—lining cells, cb—compact bone. Scale bars: A—100 μm; G—200 μm; insert in G—1 mm; C, D, E, F, and I—50 μm.”
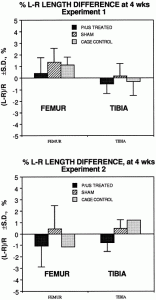
” At 3 and 4 weeks postoperation[induction of a fracture], the mature callus was composed mainly of a cancellous bone undergoing transformation into the compact bone with little if any cartilage or woven bone present. Vascularized periosteal tissue was reduced and covered the center of the callus and was entrapped within the newly formed bone. Multiple undifferentiated cells within this tissue continued to show CMF608-specific hybridization signal”
“in situ hybridization results suggest that in adult rat bones, activity of the CMF608 gene is confined to a certain subset of skeletal progenitor cells.”
“The presence of a signal peptide, the absence of a transmembrane domain(s), and the presence of structural domains typical for extracellular proteins (leucine-rich and Ig-like repeats) predict that the CMF608 protein product should be secreted out of the cell.”
“[An] important structural feature, present in all the CMF608 orthologous proteins, is conserved integrin recognition RGD motif found, however, outside the 663 amino acid N-terminal fragment. No RGD sequence was identified in adlican. Integrins are signaling receptors that connect the cytoskeleton to the extracellular matrix, and are involved in mechanotransduction. Mechanical stimulation of bone cells by fluid flow induces recruitment of integrins to focal adhesions. Since CMF608 may theoretically serve as a secreted integrin ligand and its expression is responsive to mechanical stimulation, participation of CMF608 in integrin-mediated bone-specific mechanotransduction may be anticipated.”
“Progenitor cells expressing CMF608 have a fibroblast-like morphology and are found at several locations throughout the bone sections. The lack of CMF608 expression in pluripotent C3H10T1/2 mouse mesenchymal progenitor cells allows us to suggest that CMF608 expressing fibroblast-like cells represent already committed skeletal progenitors, whereas their specific localization hints to their involvement in bone modeling by taking part in circumferential growth of the physis (cells within perichondral ring) and bone shaft (endosteal and periosteal cells of diaphysis) as well as the involvement in the reshaping of metaphysis into bone shaft.”<-so less differentiated progenitor cells can be induced to express CMF608 thus becoming growth plate progenitor cells.
In the study Superior Osteogenic Capacity of Human Embryonic Stem Cells Adapted to Matrix-Free Growth Compared to Human Mesenchymal Stem Cells, CMF608 is expressed in higher levels in embryonic stem cells than human mesenchymal stem cells.
The growth plates does seem to need a specialized stem cell from the Ring of La Croix as no other growth occurs if the ring of la croix is disrupted from delivering cells to the growth plate. However, there is a specific gene known as CMF608 that can be activated by mechanical loading or fracture. So it is possible to create these stem cells and it is possible that a LSJL type loading regime can induce the CMF608 gene.
Also the presence of CMF608 in regions other than the ring of LaCroix such as the periosteum, Volkmann’s canal and endosteum suggests that other cells can be used to form new growth plates other than specific zone of LaCroix cells.
Also, a potential height increase method could be to inject CMF608 positive stem cells underneath the periosteum to form newo-growth plates.
If you look at this image of the groove of Ranvier(A) and zone of La Croix(b) you can see that the growth plate chondrocytes seem to spill out of the groove of ranvier.
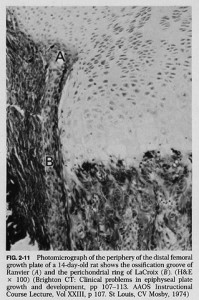
According to one site, “On the sides of the growth plate (physis) the Ossification groove of Ranvier provides cells for growth in width. The Fibrous Ring of La Croix lies outside the physis and keeps the cells from oozing out under axial loading”