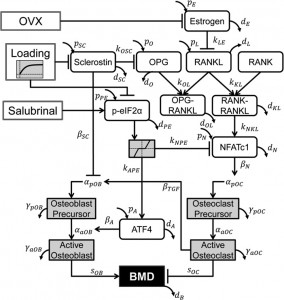
Cyclic Mechanical Shear Compression Induces Progenitor Mesenchymal Stem Cells Towards Chondrogenesis – Breakthrough!
I wanted to make one last, final extremely detailed post on the subject on what Tyler has been promoting for more than half a decade, which he has termed the Lateral Synovial Joint Loading Technique for lengthening long bones. This will be the last large post I ever intend to do since there is just too many areas of medical fields I would still need to touch and look into.
There seems to be new studies and evidence I’ve found which gives credibility to his claims. I interviewed Tyler almost more than a year ago for the 2nd podcast episode (Available Here), before he joined the website, about how he discovered and tried out this technique. He said that his finding of the studies on loading of lab mice knees and elbows by Ping Zhang and Hiroki Yokota was a turning point in his decision. He thought that after he broadcasted the study in a large enough numbers through his blog, the study and what it seemed to imply based on his opinion was that it would go viral. Well, it didn’t.
He took it upon himself to do the loading, to see what would happen. He got results. He claimed that he had gained around 1.5-2 inches of height, from a starting height of 5’7″-5′ 8″ to 5′ 10″ over his many years of using a C-Clamp to load his knees and other joints. Only recently there seems to be some major concern when he gave us an update on his progress “Height Increase Progress Update“) and he said that a recent visit to a doctor’s office and being measured by a nurse showed that he was 5′ 8.25”. In previous years, other nurses measured him at 5′ 9.75″ multiple times so I am not sure what to believe.
Multiple studies I found recently (or ones which I’ve found before but never went into deep into) suggest that from a theoretical point of view, based on studies done in a lab culture aka in-vitro, his claims can possibly work.
- Cyclic, mechanical compression enhances chondrogenesis of mesenchymal progenitor cells in tissue engineering scaffolds
- A combination of shear and dynamic compression leads to mechanically induced chondrogenesis of human mesenchymal stem cells
- Chondrogenesis of Human Bone Marrow Mesenchymal Stem Cells in Fibrin–Polyurethane Composites Is Modulated by Frequency and Amplitude of Dynamic Compression and Shear Stress
- Differential Response of Adult and Embryonic Mesenchymal Progenitor Cells to Mechanical Compression in Hydrogels
- Cyclic hydrostatic pressure enhances the chondrogenic phenotype of human mesenchymal progenitor cells differentiated in vitro
The honest truth is that Tyler has already read over all these studies at some point in the years of research he has done, and from probably looking over the abstracts, got the main point from the studies, just like what I am doing. What I am going to state in the next few paragraphs, Tyler has already said dozens of times before, but this post will be a complete outline and summary of his entire thesis, based on connecting the dots using PubMed studies. I don’t think he would disagree on any of the major points I will state below, but I do welcome in him giving his own suggestions on improving this post. It will be one of the major, seminal posts which changes the direction of the website/blog going forward.
The readers can definitely read over the studies, or even buy the entire studies but here is what I managed to take away from the abstracts and some of the studies which gave the full study.
Takeaway #1: Compression aka Loading aka Pushing down on lab-grown scaffolds (whether fibrin-polyurethane composite) with MSCs embedded inside them turns the MSCs towards the chondrocyte lineage.
Takeaway #2: Shear Compression, which is just pushing on the object you are analyzing from the sides, is another way of saying Lateral Loading. It works just as well as all other forms of compression to turn the progenitor stem cells into chondrocytes.
That is something which I would assume most amateur, somewhat knowledgeable height increase researchers agree is viable, and most likely true. Anyone who has ever read more than a dozen full length PubMed studies in trying to find the solution would understand what I am talking about.
The main concern which I will now try to resolve is this “How does induced chondrogenesis of the MSCs inside the epiphysis lead to the long bones becoming longer?”
First, we know that there is no more epiphyseal cartilage to work with. That is a given.
So, what is left? It is just the hyaline articular cartilage at the ends of the long bone.
For the person who can follow along here, the obvious next question would be “Does that mean that the articular cartilage at the ends are somehow turning into a new pseudoepiphyseal cartilage which can “grow” in a way to make the overall bone longer?”
My answer to that is “YES”.
Now, let me give you guys a little bit of background on where my main concern had been for so long. There have been many posts I’ve written looking at the validity of LSJL. They include…
Here is the basic problem I had asked of him, multiple times
The human bone is extremely hard, with material strength at the level, and even exceeding, stainless steel. The main composition found in bone which gives it such a hardness is the cortical bones, which gets the toughness from the calcium crystals embedded into the extracellular bone matrix. Those crystals are non-living, non-organic compounds in the bone.
From a diagram of a femur/tibia/humerus/long bone in the human body, at the center is a cavity. That cavity is reasonable in thickness aka width. It is so wide that the way to do internal limb lengthening surgery is to put a metal rod in the cavity. From a measurement perspective, I estimate the average Caucasian American human adult male is about 200 lbs, 5’10’-6’2″, and his femur, the largest and strongest of all the bones being able to resist compressive loading is about 4-5 cm thick or around 1.25-1.750 inches thick, (assuming that he is in his 30s and the periosteal growth has started to make his long bones wider). The intermedullary cavity is about 1-1.5 cm wide. We then consider the thickness of the trabecular bone area and maybe even add in the thickness of the periosteum, which could be just 0.25-0.5 mm at most. I have no medical studies to reference but let me guess that the thickness of the trabecular bone layer inside the metaphysis and the epiphysis of the adult male femur is about 0.5 cm thick.
Modeled out completely by drawing…..
l <—cortical bone —-> l <—–trabecular bone —-> l <— intermedullary cavity —> l same
To calculate the overall thickness of the cortical bone layer
(4 cm total thickness) – (1.25 cm of cavity thickness) – (1 cm of total trabecular bone) =
1.75 cm of cortical bone
Second Calculation: (for the large thicker area of the femur)
(5.5 cm total thickness) – (1.5 cm of cavity thickness) – (1.5 cm of total trabecular bone) –
2.5 cm of cortical bone
What does the calculation show?
That the cortical bone layer is about 1.75-2.5 cm of cortical bone thickness. Of course, this refers to a a circular shell calculation, so divide that value by half. It comes out to show that the cortical bone layer is on average from 0.875-1.25 cm thick on both sides.
What does the thickness of the bone layer tell us?
It is the exact reason why I raised so many questions to Tyler in our first Q&A email exchanges. “How does any induced chondrocytes in the head/bulb/epiphysis of the long bone manage to push against the 1.25 cm thick cortical bone layer to make the head bigger so that the overall bone becomes longer???”
The chondrocytes inside the bone will be surrounded from all 6 directions by the cortical layer, which is too strong. There is NO WAY IN HELL any group of induced chondrocytes would have the strength to push against the calcium crystal in the bone ECM!!
I never could wrap my brains around this one, main issue. That is, until I saw something today which seems to make everything I have read about make sense. I think I have finally figured out how to connect all the dots together, to explain why Tyler’s theory, this LSJL he has been talking about, could be reasonable.
We have to first look at the pictures and diagram of articular cartilage in medical textbooks and medical references. Let’s look at all the pictures and diagrams we can find on the internet on the layering of the articular cartilage, because it is not a simple, single layered tissue like so many people believe it would be.
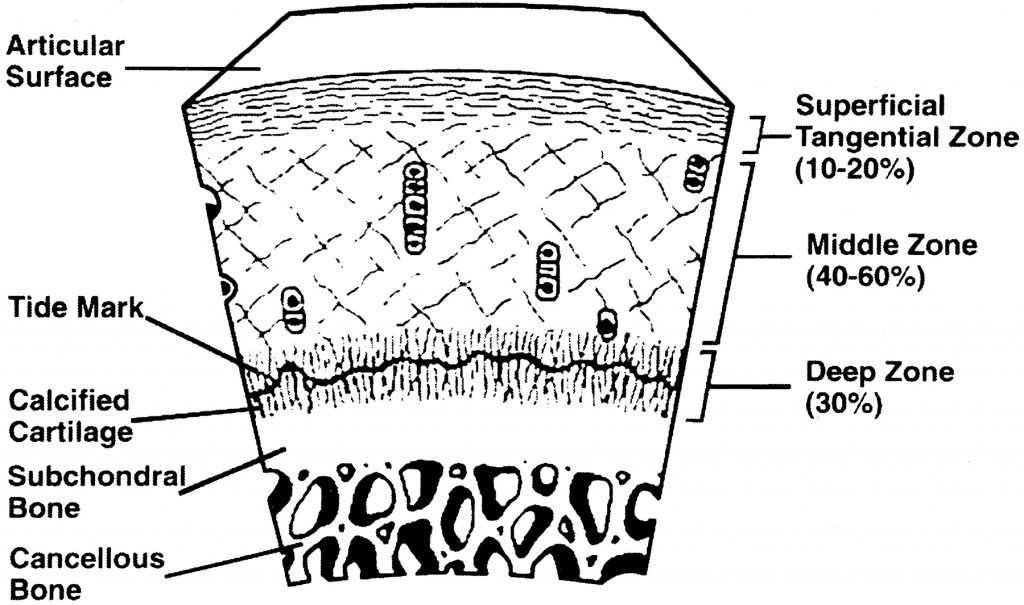
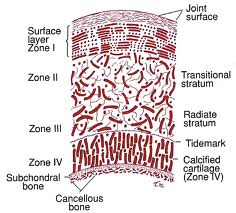
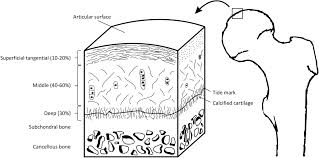
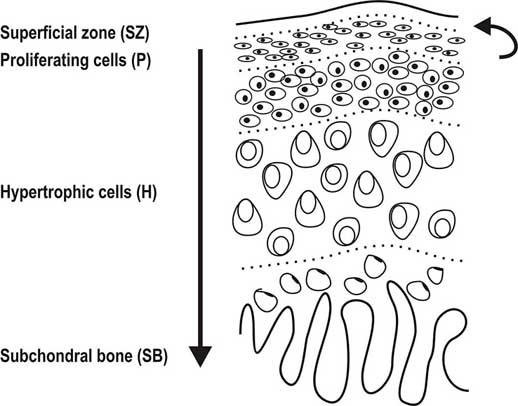
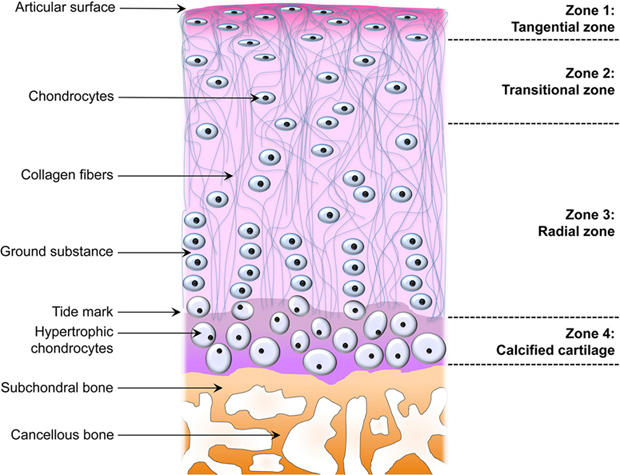
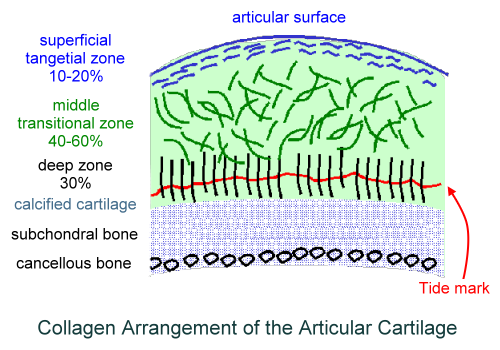
Do you guys see it???
I looked on Google Images for the best diagrams and pictures of the exact structure and orientation of the cells inside the articular cartilage, and pasted them all in one location. What do you guys notice about the articular cartilage, which is so unique?
Answer: It is the way that the articular cartilage is also stratified into multiple layers, just like the epiphyseal cartilage layer.
First, you have the articular surface, and then the superficial tangential zone. I would guess that if we did a histological analysis on what cells or fibers are actually in the tangential zone, it would turn out that the surface has thin strand like collagen fibers or fibrils. There is probably almost no chondrocytes, but plenty of lamellar oriented collagen fibers.
Then you have the area which is called either the transitional zone or the middle zone. There is chondrocytes there, which are of small size and volume. They don’t seem to be in any type of specific orientation.
Afterwards, you have the area called the deep zone which some diagrams have called the radial zone. In this area, the chondrocytes seem to have started to start to orient themselves in columns, which is very surprising. It makes no sense in my opinion for articular cartilage to orient themselves in columns What could be the purpose of change towards column like structures as we study the articular cartilage and go down deeper through the layers?
The last three layers, going down are….
- The calcified cartilage layer – If we compare the model of the articular cartilage to the epiphyseal cartilage area, this would be the layer probably which is half calcified.
- The subchondral bone layer – This is the hard cortical bone
- The trabecular/cancellous bone layer – This is the softer, weaker in material strength bone type.
What is most interesting, and what I have been focused on is the 4th layer, going from top to bottom, the calcified layer. I note that all the pictures I have uploaded are of the articular cartilage layer, NOT the epiphyseal layer!!
There are differences in the diagrams. Some of the diagrams suggest that either in the deep/radial zone, the chondrocytes also go through hypertrophy, or it would be in the calcified cartilage layer. However, the important thing to take away is that chondrocytes do seem to go through hypertrophy as well. This agrees/validates a previous post I had done a year ago entitled “Articular Cartilage At The End Of Epiphysis Do Growth Thicker Making Bones Longer (Big Breakthrough)“. I wrote that post after finding in a 1st year medical school curriculum anatomy & physiology textbook that the articular cartilage can go through a type of growth known as appositional growth and get thicker over time.
What seems to be agreed upon by the orthopaedics is that there is something known as a real boundary in the calcified layer, known as the tide mark. The anything below the tidemark is the beginning of the subchondral bone, which I have believed for a long time is make of cortical bone tissue and ECM structure.
The Tidemark of Articular Cartilage in the Diagrams
I had to look at the section in Wheeless’ Textbook of Orthopaedics website on articular cartilage to figure out what is this tidemark that is showing up. There is only a single sentence where they talk about it “tidemark is basophilic line which straddles the boundary between calcified and uncalcified cartilage”
(Edit: I still need to do much more research on the tidemark to edit this section.)
Here is where the good news start, based on a few implications/assumptions I have to make first….
Implications #1
Assume first that the 5-6 pictures I have uploaded above are on average, on the right scale. Sometimes, but especially in biological, medical, and astronomy textbooks, distances are not drawn to actual scale, to magnify smaller regions, relative to the much larger regions and areas. (For example, most young kids have built a replica of the Solar System before with the sun in the middle, but if they tried to build everything to the correct relative scale, Neptune, would need be 300 feet away from the sun, even if the sun was just 1 inch in diameter.) Most of the pictures show that the layer of subchondral bone, which would have the highest material strength if we tested it using a loading/tensile/compression machine, to be very thin. That means that at least on the very edge of long bones, at the ends, the cortical bone layer might not be as thick as on the sides. Notice how in most of the histological drawings the subchondral layer is always drawn to be thin, while all the other layers, are drawn to be thick in comparison.
Implication #2:
If the subchondral layer is thin enough, you can cause microfractures, like would be like small tunnels/ravines/crevices which will go along the layer. The microfractures can be induced by lateral loading, like a forceful squeeze of a large enough C-Clamp to get around the knee.
Example: Imagine squeezing a watermelon, or pumpkin from the sizes. Forget the stem in the center on the top and assume the fruit does not have a thick stem. With consistent, but intermittent pushing on the sides, the first onset and occurrence of damage to the outer strong shell, would be on either the top or bottom, if you do it correctly.
This is what be what will get around my original issue with LSJL. The top of the proximal tibial epiphysis could be thin enough, such that lateral loading would cause the layer to develop many microfractures in the form of deep crevices.
Implication #3:
If there is induced microfractures in that thin subchondral layer, some of the MSCs that would still from the bone marrow in the inner core of the epiphysis (made of yellow type adipocyte derived stem cells mostly) can possibly seep upwards, into the deep/radial layer of the articular cartilage. Tyler in his old blog has already written dozens of posts showing that lateral loading causes MSCs to differentiate into chondrocytes. All his diagrams on how one molecular ligand or gene expressed protein would stimulate and/or inhibit another was never completely mapped out, but he has more than validated the idea that lateral loading induces almost all the correct molecular mechanisms towards chondrogenic differentiation, proliferation, and hypertrophy.
Since the articular cartilage at the bottom has the columnar formation just like the epiphyseal layer, the new chondrocytes can proliferate, and hypertrophy some more, causing the articular cartilage to increase in thickness and/or deposit a slight layer on calcified chondrocyte layer. That is how you increase in height, from articular cartilage layer thickening due to a layer of chondrocyte deposition and hypertrophy.!!
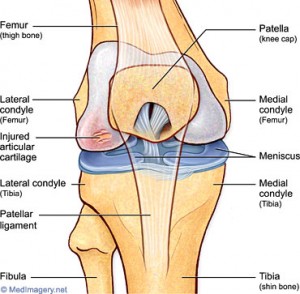 If the picture/diagram of how the articular cartilage at the end of the proximal epiphysis of the tibia in the human skeleton is even remotely accurate, then the cartilage layer just deposits a thin layer of ossified cartilage at the bottom, and the layer on top stays about the same.
If the picture/diagram of how the articular cartilage at the end of the proximal epiphysis of the tibia in the human skeleton is even remotely accurate, then the cartilage layer just deposits a thin layer of ossified cartilage at the bottom, and the layer on top stays about the same.
The process would be very slow, and the gains will be very small, but theoretically, if you inject maybe some extra chondrocytes, MSCs, or IGF-1, the original thickness of the layer stays about the tame, and you can make the tibia longer for a long time just building on the layers by depositing more layers at the bottom.
Implication #4:
The entire premise of the first part of the blog post was to show that what Yokota and Zhang had done, with the adult and mice rats, the intermittent cyclic mechanical shear compression can cause MSCs (wherever they are derived,) to become chondrogenic. That was the whole purpose of the first part.
Implication #5:
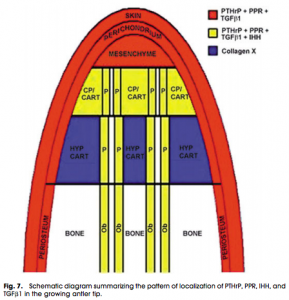 More than a year ago, I looked at how the mechanics worked for deer antlers, which fall off and regrow again. The way that deer antlers manage to grow in length is because the tip of the antlers have a storage of progenitor stem cells. When the storage at the tip of the antler is used up, the antler can no longer get any longer. Refer to the post “The Connection Between Regenerating Deer Antlers and The PTHrP, PTH And IHH pathway for Cartilage Regulation, PTHrP Seems To Be The Answer (Big Breakthrough!)“
More than a year ago, I looked at how the mechanics worked for deer antlers, which fall off and regrow again. The way that deer antlers manage to grow in length is because the tip of the antlers have a storage of progenitor stem cells. When the storage at the tip of the antler is used up, the antler can no longer get any longer. Refer to the post “The Connection Between Regenerating Deer Antlers and The PTHrP, PTH And IHH pathway for Cartilage Regulation, PTHrP Seems To Be The Answer (Big Breakthrough!)“
We can almost imagine and model the tip of the deer antlers like the layer of articular cartilage, since there is a very small bit of mesenchyme that seeps into the articular cartilage from the epiphysis from lateral loading, making it larger and longer.
Implication #5:
In a critical post I had written about a month ago, I had given a theory on how the mechanism of angled LSJL technique would work. Dr. Robert Becker revealed that if you bend bones in one angle, thicker bone would deposit on the side of the bone that is being compressed. This was theorized to be due to the movement of electrons which would be popped out of the calcium crystals, which then flow through the bone, to the area, which is being compressed. The excess negative charge, from the electrons, would draw the positive cations from the bone towards them, causing the calcium crystal density in that region of the bone to increase. Refer to the post “Why LSJL Could Work And What We Have Been Doing Wrong, Thank You Nixa Zizu – Big Breakthrough!“
That post was the start of a completely new way of imagining how LSJL should be done.
Conclusion
All the research that Tyler has been doing for almost a decade now, his claims actually seem to be valid based on at least the theory out there right now. It is theoretically viable and his claims now finally make sense. I think that I have covered every aspect of how the mechanical process would work out, if it succeeds.
So how come there have been so few people who did LSJL have succeeded?
While the theory now on how LSJL could work, even for adults with not epiphyseal cartilage, finally makes sense, we still have to resolve the issue on why so few people get results. My guess right now is that we have been clamping in the wrong angle, in the wrong location, and we don’t give enough time for the synovial joints to get the MSCs inside the articular cartilage to go through hypertrophy and deposit on the bottom layer.
There is also the fact that young people in their early-mid 20s (who do have fully ossified growth plate cartilage), have probably healthier articular cartilage tissue to begin with, so the nature of the tissue is just more malleable and more responsive to any type of mechanical stimuli.
A Message from Michael/Admin: Like I said before, this will be the last in-depth post I will ever write about the Lateral Synovial Joint Loading Technique. I feel like that I have finally been able to prove using histomorphological analysis of the articular cartilage layer that the technique that Tyler has been promoting for so long does make sense theoretically. I wish to move on to other areas of research. This subject is more for him. I hope other people can leave some feedback and comments on what they think. Do they think that this post finally answers the question on the efficacy of LSJL, with an affirmation?
 Recently I went back on the kick to learn medicine again, and I was told by a post/article written on the medical website KevinMD (Available Here) to read the following three medical memoir type books, which supposedly most medical students have read, or will read at some point. So I bought two of them through Kindle and started to read over them. The books are…
Recently I went back on the kick to learn medicine again, and I was told by a post/article written on the medical website KevinMD (Available Here) to read the following three medical memoir type books, which supposedly most medical students have read, or will read at some point. So I bought two of them through Kindle and started to read over them. The books are…