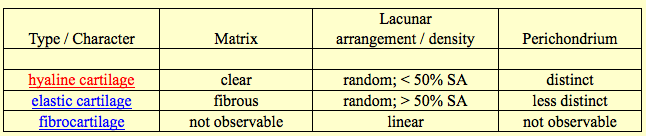
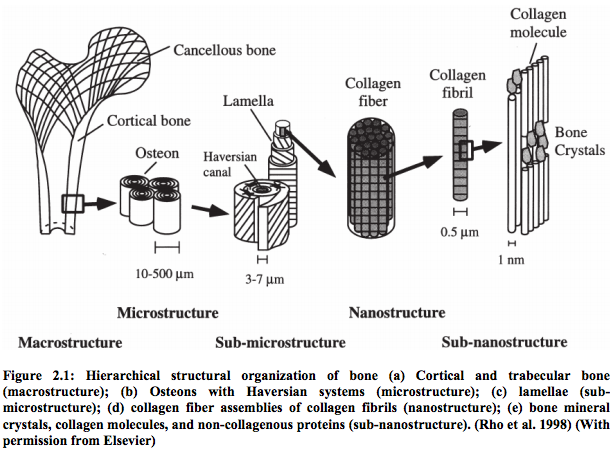
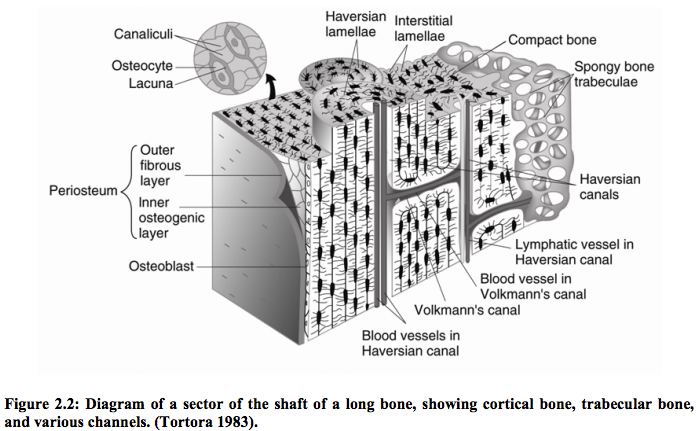
I think this may be the first study I have found where there is something substantial to show for from doing so much research.
These groups of university grad students, post docs, and professors have finally, FINALLY been able to engineering growing cartilage tissue in vivo through chondrocyte transplantations. The result is that they have been able to engineering growing cartilage that exhibit the same type of characteristics as the epiphyseal growth plates.
The only thing that it doesn’t say is that they can do this non-invasively. However this type of ability is a huge breakthrough in terms of the research we have been dedicated to.
The paper below shows that bones with cartilage attached can grow like the natural endochondral ossification has been emulated
University PDF Article: Engineering growing tissues
Authors: Eben Alsberg, Kenneth W. Anderson, Amru Albeiruti, Jon A. Rowley, and David J. Mooney
University of Michigan, Ann Arbor, MI 48109
Edited by Robert Langer, Massachusetts Institute of Technology, Cambridge, MA, and approved July 10, 2002 (received for review May 15, 2002)
Abstract
Regenerating or engineering new tissues and organs may one day allow routine replacement of lost or failing tissues and organs. However, these engineered tissues must not only grow to fill a defect and integrate with the host tissue, but often they must also grow in concert with the changing needs of the body over time. We hypothesized that tissues capable of growing with time could be engineered by supplying growth stimulus signals to cells from the biomaterial used for cell transplantation. In this study, chondrocytes and osteoblasts were cotransplanted on hydrogels modified with an RGD-containing peptide sequence to promote cell multiplication. New bone tissue was formed that grew in mass and cellularity by endochondral ossification in a manner similar to normal long-bone growth. Transplanted cells organized into structures that morphologically and functionally resembled growth plates. These engineered tissues could find utility in treating diseases and injuries of the growth plate, testing the effect of experimental drugs on growth-plate function and development, and investigating the biology of long-bone growth. Furthermore, this concept of promoting the growth of engineered tissues could find great utility in engineering numerous tissue types by way of the transplantation of a small number of precursor cells.
Analysis #1:
I’m going to treat this post a little different due to nature of how critical this study is in our endeavor. This study and paper is a big game changer. That is why I will be doing a series of smaller, shorter analysis for each section I post to show the reader what the implications are. These researchers at the University of Michigan, Ann Arbor, from the Department of Biomedical Engineering have been able to do a 5 step process
1. Buy the raw materials needed to make the medium, which is a alginate based hydrogel.
2. Embedded a peptide that they bought and modified into the hydrogels first. These peptides have a specific type of animo acid chain sequence that is arginine-glycine-aspartic acid which is also called R-G-D, RGD. The longer peptide chain is actually G-G-G-G-R-G-D-Y (aka G4RGDY)
3. They take a subject/patient and remove some bone and cartilage cells from them, which are called the osteoblasts and chondrocytes. This is the explant.
4. They put the osteoblasts and chondrocytes into the now already embedded hydrogel.
5. The hydrogel is placed in an area of a lab animals (for this case, it is mice) skeleton where there is bone/cartilage missing.
The thing that they have shown is that not only have they been able to create bone tissue (old news), or cartilage tissue (harder, but still doable), but growing bone tissue, which means that the cartilage cells that is with the bone cells is proliferating, hypertrophizing, and turning out cartilage that is expanding.
This is exactly how the growth plates work.
From the introductions area…
We hypothesized that it would be possible to engineer a growing tissue by presenting appropriate growth stimuli from the cell transplantation scaffold. This hypothesis was tested in the context of engineering growing bony tissues by the cotransplantation of osteoblasts and chondrocytes. It is critical to promote the multiplication of transplanted cells if one is to engineer a growing tissue in vivo, and one required growth stimulus for most mammalian cell types is an appropriate adhesive substrate. We hypothesized that providing a high density of adhesive ligands to transplanted chondrocytes from the polymeric delivery vehicle would promote their multiplication, and synthetic peptides containing the arginine-glycine-aspartic acid (RGD) cell adhesion sequence were covalently coupled to the alginate polymer chains used to form the hydrogel delivery vehicle to provide this requirement. We have previously documented that this approach allows one to specify the mechanism of cell–material adhesion, and that an appropriate density of RGD ligands promotes the proliferation of various cell types in vitro. Considerable efforts have been made to date to regenerate bone and cartilage tissues separately and together, but no attempts to form a growing bony tissue have been reported. We now demonstrate it is possible to regenerate a tissue structurally and functionally similar to a growth plate by providing a growing cartilage anlage with transplanted chondrocytes, similar to that in long-bone development, as a framework for subsequent bone formation by cotransplanted osteoblasts.
Analysis #2:
These researchers felt that to get the chondrocytes to grow cartilage that will expand again, they will add/embed the growth factors first into the hydrogel matrix/scaffold.
Note 1: Most tissue engineering is already done this way. The idea of first embedding growth factors is the standard approach, not something new or totally radical.
Note 2: When the researchers are using the terms, alginate, scaffold, matrix, or hydrogel they are talking about the exact same thing. So alginate = scaffold = matrix = hydrogel
The cells are then added next into the hydrogel and over time, the already embedded peptides will diffuse or seep into the cells and get them to proliferate and eventually disintegrate the hydrogel matrix and leave a cartilage matrix in its place.
They get it right in focusing mainly on the need to have the chondrocytes that they transplanted to focus mainly on multiplication/division/proliferation. To make sure that the cells are doing this, they need to add growth stimuli. One of the growth stimuli that they have found from their research which works is an adhesive substrate, at least for mammalian cells. They wanted to test the idea of taking a high density of these adhesive ligands, which are synthetic peptides containing that RDG amino acid sequence, and getting them to bond to the chondrocytes and obsteoblast’s membrance surface. It seems that the specific type of peptide sequence can specify the mechanism of cell adhesion. If the RGD ligands are at the right density, they can promote the proliferation of various types of cells in vitro.
The introduction is concluded by the researchers stating that growing bones which are going through the endochondral ossification process has never been successfully created. It seems that they may be the first group of researchers that have succeeded and published a paper showing their results.
Results and Discussion, Part 1 -(By the researchers, In the article)
To test first if a growing cartilaginous tissue could be engineered, as a first step to engineering growing bony tissues, RGD-modified and unmodified alginate hydrogels were used to transplant isolated chondrocytes into mice for periods from 6 to 25 weeks. Gross examination of explanted tissues revealed that only implants combining chondrocytes with RGD-alginate demonstrated convincing characteristics similar to native cartilage (e.g.,pearly white opalescence, firm to palpation) (Fig. 1a and b). Furthermore, tissues engineered with the RGD-modified alginate increased significantly in mass (Fig. 1c) and size over time. Quantification of the cell number in these tissues indicated a continued increase in cell number over time (data not shown), supporting the gross observation of extensive tissue growth.
Analysis #3:
Again, like I said and the researchers said before, there were three main groups, the negative control, the control and the experimental. Two groups had explanted chondrocytes that were grown in in culture up to a certain cell chondrocyte concentration. two groups had the alginate/hydrogel/scaffold put into them. Two groups had the chondrocyte seed into the scaffold. Only the experimental group had the RGD added into the alginate beforehand. The result is that the RGD-modified alginate that was put into the experimental group caused the chondrocytes to grow into cartilage that had the right color and firmness. The tissues as a result increased in both mass and size over time. This means that whatever type of tissue did grow, they increase in volume, (aka REAL BONE VOLUME GROWTH). More testing showed that the cells had indeed increased in number indicating that they were proliferating.
Results & Discussion, Part 2
Histologic evaluation of the engineered tissues indicated an accelerated rate of tissue formation (e.g., higher cellularity and type II collagen deposition, decreased residual alginate) with the RGD-alginate hydrogels. The negative control group (unmodified alginate without cells) demonstrated only residual alginate and fibrovascular ingrowth; no evidence of cartilage formation was exhibited by any of these implants at any time. Implants of unmodified alginate with chondrocytes demonstrated islands of cartilage-like tissue (Fig. 1d) that were noted to expand and coalesce with increasing time of implantation. In contrast implants of RGD-alginate with chondrocytes demonstrated an abundance of cartilage-like tissue even at the earliest time point, with small islands of residual alginate contained within the cartilage (Fig. 1e). The ratio of cartilage to alginate increased with time, and at the 25-week time point these implants were almost entirely composed of cartilage-like tissue, with only occasional small pockets of residual alginate. Quantification of the areas staining positive for type II collagen (a specific marker for cartilage), by using computerized image analysis, revealed that the negative control demonstrated no type II collagen deposition. In contrast, the RGD-alginate group exhibited extensive positive staining (95 plus and minus 3% of tissue area). These tissues also demonstrated significantly greater compressive moduli than tissues engineered with unmodified alginate (data not shown), further supporting the finding of increased rate of cartilage formation. Significant progress has been made toward engineering functional cartilage tissue in terms of achieving mechanical, biochemical, and histologic properties similar to those properties of native cartilage (17); however, no growing cartilage tissue has been reported previously. The current findings demonstrate that providing specific cell-adhesive interactions with the cell delivery vehicle can markedly enhance the growth of engineered cartilage tissue.
Analysis #4:
This part reiterates the point that with the negative control, which has neither chondrocytes of RGD peptides implanted, there was no chondrocytes or cartilage signs over time. With the control group where there was chondrocytes but no RGD there were pockets of chondrocytes around but the cartilage/alginate ratio didn’t change much over time. With the RDG-alginate group, there was a clear sign that Collagen Type II production was occuring, as well as an increased cartilage/alginate ratio, which kept going higher. Eventually there was little alginate left, but just filled with cartilage. The cartilage that did develop seemed to also have a high compressive moduli, showing that even the firmness and strength that are seen in natural growth plates are shown in the RGD-alginate group.
Results & Discussion, Part 3
Once a growing cartilage template had been achieved, the cellular environment present during endochondral ossification was partially recreated in an effort to form a tissue engineered growth plate-like structure. Past studies aimed at modeling growth plate physiology used monolayer chondrocyte cultures, which do not stabilize the chondrocyte phenotype and neglect the true in vivo spatial arrangement of chondrocytes and their matrix (18, 19). Subsequent studies have used chondrocyte aggregates (20) or suspension cultures in which chondrocytes were cultured in a variety of three-dimensional gels (21–27). Although these systems preserved the chondrocyte rounded phenotype and provided a more realistic three-dimensional environment, none of these models provided for chondrocyte transformation from a proliferative to a differentiating phenotype within the growth plate (20) nor accounted for the complex cell–cell interactions and soluble signaling that occurs between osteoblasts and chondrocytes within an actual growth plate. To address these limitations of past models, chondrocytes and osteoblasts were mixed together in a G4RGDY-modified alginate delivery vehicle and injected into the backs of severe combined immunodeficient mice for 4–26 weeks. A control of osteoblasts-alone transplantation was also used in this study. Engineered tissues were excised at 26 weeks, and gross inspection of both experimental groups revealed the appearance of bony nodules (Fig. 2a and b). Specimens with a 2:1 ratio of primary RCO to primary BAC cells were substantially larger than the RCO-only transplants, however, and also had regions that grossly resembled cartilage. Bone mineral density plots taken with dual-energy x-ray absorptiometric imaging on implants at 26 weeks confirmed mineral content throughout the implants in both groups (Fig. 2c and d). Although the bone mineral density was significantly greater in the RCO-only group than in the cotransplantation group at 13 and 26 weeks (Fig. 2e),the bone mineral content in the cotransplantation group was significantly greater than that of the RCO-only group at 4 and 26 weeks (Fig. 2f). In addition, the mass (Fig. 2g) and the cell number per implant (Fig. 2h) of the cotransplantation group significantly increased over time, while no increase in either variable was observed in the RCO cell-only condition over time. Thus, cotransplantation of chondrocytes and osteoblasts in this vehicle resulted in a growing bony tissue, as evidenced by significantly increased mineral content, mass, and cell density over time.
Analysis #5:
I think this is the section which really reveals why this study is such a big game changer.
1. Researchers have tried to grow from scratch a functional growth plates with similar characteristics as the natural thing. They had tried to go with a monolayer structure. This approach was not successful because the form or shape of the chondrocytes could not be stablized and sustained. The other big problem was that the monolayer was not really representing how the chondrocytes are aligned spatially in real growth plates.
2. Other researchers tried to put the chondrocytes into a packing form in 3-D gels and cultures. These did solve the problem on getting the chondrocytes to stay in their rounded form as well as get the structural alignment of chondrocytes relatively similar like real growth plates. However these research found out the problems that arose for them was that the chondrocytes didn’t differentiate in the way (ie. Hypertrophize) that real growth plates would do. Another problem was that the 3-D suspensions could not account for the cell-cell signaling that was done between the bone layer and the cartilage layer during ordinary endochondral ossification.
3. The researchers who wrote this article tried to account for all of the 4 major problems by putting osteoblast progenitor cells in with the chondrocyte progenitor cells. They did the bone-cartilage cell combination along with a control group of just using bone cells. The results showed that there were bony nodules. The ones with cartilage cells in the mix showed at the macroscopic level to be cartilage like. The bone mineral density of the bone cell transplant was higher but the cartilage-bone cell cotransplant showed that there was a higher cell density and the volume of the bone that resulted was bigger.
Results & Discussion, Part 4
Histologic examination of the RCO cell only and cotransplantation constructs revealed mature bone formation in both conditions at 26 weeks (Fig. 3a and b). The cartilage, however, was present only in the cotransplantation group, as confirmed both morphologically and through specific staining of sulfated mucopolysaccharides (component of cartilage) (Fig. 3c and d). Histomorphometric analysis of hematoxylineosin and aldehydefuchsin alcian blue eosin-stained sections, by using image analysis software, revealed that the total amount of bone achieved with cotransplantation of the two cells types was significantly greater than that obtained from the transplantation of RCO cells alone (Fig. 3e). In addition, unlike the RCO-only group, the cotransplantation group demonstrated significant cartilage tissue and marrow space. Minimal alginate remained in the cotransplantation group, whereas more than 50% of the tissue in the RCO-only group was residual carrier.
Analysis #6:
Testing showed that the volume size of the bone that resulted from the cotransplantation was much bigger than just using the bone cells as a transplant. In addition, the cotransplantation caused most of the alginate/scaffold to be either disintegrated or absorbed while a a signifiant amount of the alginate was still around for the RCO-only group.
Results & Discussion, Part 5
A striking result observed in the cotransplantation group was that 80% of these growing bony tissues contained structures that histologically resembled growth plates (Fig. 4a and b) at the interface of the cartilaginous and bony regions of the tissue. The first region of these structures is similar to the reserve zone of normal growth plate histology, where the spherical chondrocytes exist individually or in pairs and are similar in size to the cells of the proliferative zone (Fig. 4c). These chondrocytes are separated by large amounts of extracellular matrix and not as densely packed as cells in the other regions (28). In the next region, the chondrocytes, flattened and aligned in longitudinal columns, mirror the morphology of the proliferative zone of the growth plate (Fig. 4 c) (29). The flattened cells of the preceding region turn into rounded, distended chondrocytes, which are similar in morphology to the hypertrophic zone of a growth plate (Fig. 4d). The final region exhibits trabeculae of primary spongiosa and marrow space typical of a growth plate’s metaphysis (Fig. 4e) (3). The regions of cartilaginous and bony tissues ranged in size from 1.75 to 11.50 mm 2, with the junctions between the zones ranging from 1 to 6.5 mm. Organization of transplanted cells to form growth-plate-like structures has not been reported, but other cell types (30–36) have demonstrated the ability to self-assemble in vitro into structures that have similar functional and or morphological properties to the tissues from which they were isolated (37). In addition, several groups have engineered complex functional tissues such as the urinary bladder (38) and the small intestine (39) through the transplantation of multiple cell types in specific locations on the delivery vehicle. However, the development of growth-plate-like structures presented here is a striking demonstration of the ability of randomly mixed multiple cell types to self-organize into several distinct tissue types. The mechanisms of cellular self-assembly underlying these findings are not entirely understood, but may include differential adhesion between different cell types, differential response to chemotactic gradients, different rates of adhesiveness reacquisition after cell isolation, the differential contractility hypothesis, and the specific adhesion hypothesis (40). The different cell types may also affect the organization of each other by the secretion of growth factors, or the cell populations may respond differently to the insoluble signals provided by the adhesive moiety bonded to the alginate vehicle.
Analysis #7:
This is the part of the article where the researchers compare what the tissue that was formed from the cotransplantation to the type of cell morphology seen in ordinary growth plates. They comment that 80% of the cells in the tissue that develops looks very much like the interface in growth plates where the cartilage meets the bone. There is a section of the tissue that resemble the resting zone, another section of the tissue that resemble the proliferation zone, and a third area which matches the hypertrophy zone of growth plates. This means that the continuous process in which chondrocytes result in expanded volume of bone has been duplicated.
The researchers again make the point to show that there has not been a report yet of any researcher being able to generate “growing bones” before…
“Organization of transplanted cells to form growth-plate-like structures has not been reported, but other cell types (30–36) have demonstrated the ability to self-assemble in vitro into structures that have similar functional and or morphological properties to the tissues from which they were isolated (37)…However, the development of growth-plate-like structures presented here is a striking demonstration of the ability of randomly mixed multiple cell types to self-organize into several distinct tissue types.”
This shows that even though the researcher can’t explain the minute details or the mechanism of just how the chondrocytes and the osteoblasts (along with the RGD-peptides and alginate scaffold) come together to create a working growth plate like unit, the histological testing shows that the tissue seems to work almost exactly like the growth plates.
Conclusion:
Months ago, around the end of last year in december I wrote a post that stated that I was 99% sure that epiphyseal growth plates have already been successfully grown in the lab. The post was “A Real Alternative To Limb Lengthening Surgery – Epiphyseal Growth Plate Regeneration, Regrowth, Implantation, And Transplantation Is Completely Possible (Big Breakthrough!)”
In addition, I also wrote a post about another study showing that if we could grow the growth plates to the right size that are needed for implantation, the surgery for growth plate implantation would have a high chance of success as long as we can get the vascularization issue resolved.
“Epiphyseal Plate Transplantation Through Vascularization (Breakthrough!)”
In addition, I wrote about the fact that China was in their military hospital doing experiments on rabbits to get the growth plates regrown
“China Military Hospital Research Clinics Have Already Engineered Functional Epiphyseal Growth Plates (Breakthrough)”
In the post above, I cited 5 articles that Chinese Military researchers have published about their results. They are extremely close, if not already successful in getting growth plate transplantation down at least, but maybe not complete growth plate generation from scratch.
Study #1: [The treatment of premature arrest of growth plate with a novel engineered growth plate: experimental studies].
Study #2: [Repair of upper tibial epiphyseal defect with engineered epiphyseal cartilage in rabbits].
Study #3: [Repair of growth plate defects of rabbits with cultured cartilage transplantation].
Study #4: [Experimental and clinical research on repair of growth plate injury].
Not only are the Chinese Military doing this type of research, researchers from Hong Kong seem to be able to show that engineered cartilage pellets that are implanted back in lab rabbits have been shown to be able to instigate longitudinal growth again. Refer to the study below…
Interestingly, these Korean researchers from Yonsei University have been able to do the same type of experiment, showing that chondrocyte allograft transplantations would work in repairing broken growth plates which might have developed body bridges.
In every one of them, the researchers used the standard tissue engineering and stem cells principles. Thinking back even further into the research of the news, I remember now that at the beginning of this website, I had found off of the Make Me Taller forum thread entitled “Russian scientists develop an alternative way of growing taller using step cells“ a link to this article about this group of researchers from Russia who had been able to implant stem cells into human leg bone to make them grow longitudinally again. The post was…“Great News For Stem Cell Method For Height Increase! :)”
The original article was entitled “RUSSIAN SCIENTISTS CREATE LEG BONE EXTENSION PRODECURE”. There seems to be 2 sources I found, Source 1, and Source 2, both telling the same story. Source 1 was from a russian news website called Rionavasti and the 2nd source was from a website called “Newlife Certified, Top Board Certified Philippine Plastic Surgery”
Note: The story by Dr. Joaquino which is the 2nd source referenced the 1st source so it turns out that there is only source for this story. It makes me question the legitimacy of the claims and of the researchers.

The ideas and techniques that are claimed to be done by these Russian scientists sound similar to already well known tissue engineering and tissue regeneration technologies. Years ago, there was a story “Sky News Exclusive: Groundbreaking stem cell technique used to repair and lengthen bones” where Scientists in the UK injected stem cells into the fracture of a broken leg. Then they used a “high tech scaffold” to stretch the stem cell filled fracture apart and then added more stem cells. The term “scaffold” is used again and it makes me think that maybe the scaffold which is implanted is the key.
There is a lot of information in this post so I wanted to state one thing for the readers of this website/blog. We have been successful in regenerating cartilage that act just like growth plates from using stem cells coupled with tissue engineering to develop bones that growth in volume.
We have also been relatively successful in being able to transplant into lab animals growth plates.
It is only a matter of time before some researchers will try to implant/embed a newly grown growth plate cartilage into the bones of an adult human being. When that happens, humans will be able to grow their bones again.