Recently I found a patent which seems to propose the idea that there might something even simpler and easier we can do to stimulate bone growth. Bone growth implies increasing bone density and possibly also accelerated rates of bone fracture healing. The simpler idea is to create food types with certain chemical inside which can stimulate bone density increase.
This inventor seems to have a patent for certain compounds they have either created or hypothesizes will stimulate bone growth from oral ingestion.
Analysis & Interpretation
The idea of the invention is to reformulate statin and other bone growth enhancing chemicals compounds into edible supplements a person can put in their food already to gain a very easy natural way of bone growth.
The thing is that the inventor is very comprehensive in their research. They admit that the BMP group has osteogenic properties but because they are peptides, they can’t be taken orally but need to be injected subcutenously (under the skin) into the blood stream. Plus, the BMP seems to be produced by different parts of the body and has effects on different parts of the body. If the BMP was taken orally, it would have an overal systemic affect on the entire body, not just the specific local areas the inventor is hoping to target.
For The Researcher, What Is The Science Behind This Claim?
The thing about BMPs are that they are produced when the precursor cells start to differentiate into osteoblasts, which are the cells which produce bone. The cells also seem to produce Type-1 collagen, osteocalcin, osteopontin and alkaline phosphatase. The inventor seems to have found that molecules or chemicals which have bone growth enhancing properties are made of this type of chemical structure…
“…Ar1—L—Ar2 wherein Ar1 and Ar2 are aromatic moieties and L is a linker that separates them by a specified distance.”
It seems however the real group of type of molecules the inventor wants to get a patent for are the “statins”. He states…
“Another class of compounds comprises inhibitors of β-hydroxy-β-methyl glutaric acid CoA (HMG-CoA) reductase. These compounds also enhance bone formation; they are generically known as “statins.” HMG-CoA reductase is the principal rate limiting enzyme involved in cellular cholesterol biosynthesis.”
There have been a few other patens which came out, specifically ones by Zymogenetics of Seattle who in the early 2000s were also developing bone growth pills and the primary compound they looked at was “statin”. What I didn’t know was that the statin was an entire group of chemical molecules. These go by the much longer term inhibitors of β-hydroxy-β-methyl glutaric acid CoA (HMG-CoA) reductase. He notes that in a few studies it was shown that lovastatin did seem to decrease osteoporosis but there was no study which showed that lovastatin directly increased bone formation. The invention would be in some type of liquid form which can be added into foods as a supplement to enhance bone growth.
 Nutritional supplements for stimulating bone growth – US 6410521 B1
Nutritional supplements for stimulating bone growth – US 6410521 B1
Gregory R. Mundy et al.
A food or food supplement which comprises a compound that enhances bone growth in vertebrates is described wherein the food or foodstuff is formulated so as to provide the desired bone growth enhancing effect.
TECHNICAL FIELD
The invention relates to foodstuffs and nutritional supplements which contain active ingredients that stimulate bone growth in humans and other vertebrates. More specifically, the invention concerns methods to enhance bone growth by supplementing the diet of humans and domesticated animals with edible materials that contain statins or other bone growth enhancers and by formulating such enhancers in edible form.
BACKGROUND ART
Bone is subject to constant breakdown and resynthesis in a complex process mediated by osteoblasts, which produce new bone, and osteoclasts, which destroy bone. The activities of these cells are regulated by a large number of cytokines and growth factors, many of which have now been identified and cloned.
There is a plethora of conditions which are characterized by the need to enhance bone formation. Perhaps the most obvious is the case of bone fractures, where it would be desirable to stimulate bone growth and to hasten and complete bone repair. Agents that enhance bone formation would also be useful in facial reconstruction procedures. Other bone deficit conditions include bone segmental defects, periodontal disease, metastatic bone disease, osteolytic bone disease and conditions where connective tissue repair would be beneficial, such as healing or regeneration of cartilage defects or injury. Also of great significance is the chronic condition of osteoporosis, including age-related osteoporosis and osteoporosis associated with post-menopausal hormone status. Other conditions characterized by the need for bone growth include primary and secondary hyperparathyroidism, disuse osteoporosis, diabetes-related osteoporosis, and glucocorticoid-related osteoporosis.
One group of compounds suggested for enhancing bone formation comprises bone morphogenic proteins (BMPs). The BMPs are novel factors in the extended transforming growth factor β superfamily. Recombinant BMP-2 and BMP-4 can induce new bone formation when they are injected locally into the subcutaneous tissues of rats (Wozney J., Molec Reprod Dev (1992) 32:160-67). These factors are expressed by normal osteoblasts as they differentiate, and have been shown to stimulate osteoblast differentiation and bone nodule formation in vitro as well as bone formation in vivo (Harris S., et al., J. Bone Miner Res (1994) 9:855-63). This latter property suggests potential usefulness as therapeutic agents in diseases which result in bone loss.
The cells which are responsible for forming bone are osteoblasts. As osteoblasts differentiate from precursors to mature bone-forming cells, they express and secrete a number of enzymes and structural proteins of the bone matrix, including Type-1 collagen, osteocalcin, osteopontin and alkaline phosphatase (Stein G., et al., Curr Opin Cell Biol (1990) 2:1018-27; Harris S., et al. (1994), supra). They also synthesize a number of growth regulatory peptides which are stored in the bone matrix, and are presumably responsible for normal bone formation. These growth regulatory peptides include the BMPs (Harris S., et al. (1994), supra). In studies of primary cultures of fetal rat calvarial osteoblasts, BMPs 1, 2, 3, 4, and 6 are expressed by cultured cells prior to the formation of mineralized bone nodules (Harris S., et al. (1994), supra). Like alkaline phosphatase, osteocalcin and osteopontin, the BMPs are expressed by cultured osteoblasts as they proliferate and differentiate.
Although the BMPs are potent stimulators of bone formation in vitro and in vivo, there are disadvantages to their use as therapeutic agents to enhance bone healing. Receptors for the bone morphogenetic proteins have been identified in many tissues, and the BMPs themselves are expressed in a large variety of tissues in specific temporal and spatial patterns. This suggests that BMPs may have effects on many tissues in addition to bone, potentially limiting their usefulness as therapeutic agents when administered systemically. Moreover, since they are peptides, they would have to be administered by injection. These disadvantages impose severe limitations to the development of BMPs as therapeutic agents.
Small molecules that are useful in treating bone disorders in vertebrates are of the general formula Ar1—L—Ar2 wherein Ar1 and Ar2 are aromatic moieties and L is a linker that separates them by a specified distance. These are disclosed in PCT application WO98/17267 published Apr. 30, 1998. These compounds were assessed for usefulness in treating bone disorders by their ability to enhance the production of a reporter protein when the nucleotide sequence encoding the reporter protein is operably linked to the promoter for BMP-2. Similar compounds are disclosed for this purpose in earlier filed PCT applications WO97/15308 published May 1, 1997 and WO97/48694 published Dec. 24, 1997.
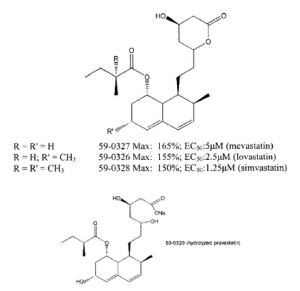
Another class of compounds comprises inhibitors of β-hydroxy-β-methyl glutaric acid CoA (HMG-CoA) reductase. These compounds also enhance bone formation; they are generically known as “statins.” HMG-CoA reductase is the principal rate limiting enzyme involved in cellular cholesterol biosynthesis. The pathway is also responsible for the production of dolichol, ubiquinones, isopentenyl adenine and farnesol. HMG-CoA reductase converts 3-hydroxy-3-methyl-glutaryl CoA (HMG-CoA) to mevalonate. Addition of mevalonate at concentrations between 25-800 μM inhibits the activity of mevastatin (100, 25, or 6.25 μM) in the ABA assay described in Example 1 herein. Mevalonic acid has no effect on primary screen activities of bone growth-active compounds outside of the statin family (compounds 59-0008 (see Example 1)). These data indicate that the effect of mevastatin in the ABA assay is mediated by its effect on HMG-CoA reductase. Knowledge of inhibitors of the cholesterol biosynthetic pathway (including SAR or pharmacophore analyses) may be useful in determining appropriate modifications or analogs of the statins that maintain bone growth activity.
U.S. Pat. No. 5,280,040 discloses compounds described as useful in the treatment of osteoporosis. These compounds putatively achieve this result by preventing bone resorption. Related to these compounds are the bisphosphonates—the methylene bisphosphonic acids. These compounds are comprised of two phosphonic acid residues coupled through a methylene linkage. Typical representatives include the clodronates which are simple compounds wherein the phosphonic acid residues are coupled through dichloromethylene. Other representative bisphosphonates include ibandronates, the risedronates, alandronates and pamidronates. These compounds have been shown to inhibit the resorption of bone, presumably by effecting apoptosis of osteoclasts. Luckman, S. P., et al., J Bone Min Res (1998) 13:581-589.
Wang, G.-J., et al., J Formos Med Assoc (1995) 94:589-592 report that certain lipid clearing agents, exemplified by lovastatin and bezafibrate, were able to inhibit the bone resorption resulting from steroid administration in rabbits. However, there is no suggestion in Wang, et al., that lovastatin directly enhances bone formation. An abstract entitled “Lovastatin Prevents Steroid-Induced Adipogenesis and Osteoporosis” by Cui, Q., et al., appeared in the Reports of the ASBMR 18th Annual Meeting (September 1996) J. Bone Mineral Res. (1996) 11(S1):S510. The abstract reports that lovastatin diminished triglyceride vesicles that accumulated when osteoprogenitor cells cloned from bone marrow stroma of chickens were treated in culture with dexamethasone. Lovastatin was reported to diminish the expression of certain mRNAs and to allow the cells to maintain the osteogenic phenotype after dexamethasone treatment. Further, chickens that had undergone bone loss in the femoral head as a result of dexamethasone treatment were improved by treatment with lovastatin. Again, there is no suggestion that lovastatin directly enhances bone formation in the absence of steroid treatment. In addition, PCT publication WO99/45923 describes methods of inhibiting bone resorption by administering an HMG-CoA reductase inhibitor. Again, there is no suggestion that statins or other HMG reductase inhibitors enhance the formation of bone.
The distinction between enhancing bone formation and inhibiting resorption is further elucidated by Ducy, P., et al., Nature(1996) 382:448-52 who have recently reported that osteocalcin deficient mice exhibit a phenotype marked by increased bone formation and bones of improved functional quality, without impairment of bone resorption. Ducy, et al., state that their data suggest that osteocalcin antagonists may be of therapeutic use in conjunction with estrogen replacement therapy (for prevention or treatment of osteoporosis).
The present invention describes means for administering statins and other classes of compounds that enhance bone formation by including them in nutritional supplements, or by administering edible materials that naturally contain these compounds. In addition, the invention is directed to an optimum protocol for administering compounds that enhance bone formation.
What is claimed is:
1. A method to enhance bone formation in a human characterized by a condition selected from the group consisting of osteoporosis, bone fracture or deficiency, primary or secondary hyperparathyroidism, periodontal disease or defect, metastatic bone disease, osteolytic bone disease, post-plastic surgery, post-prosthetic joint surgery, and post-dental implantation, which method comprises administering to said human, as a dietary supplement, an effective amount of Red Yeast Rice.
2. The method of claim 1, wherein said administering is on an intermittent protocol wherein the time period occupied by dosage administration is less than 50% of the time frame over which treatment is administered, as measured by the time between initial administration and assessment of results.
3. A method to enhance bone formation in a human characterized by a condition selected from the group consisting of osteoporosis, bone fracture or deficiency, primary or secondary hyperparathyroidism, periodontal disease or defect, metastatic bone disease, osteolytic bone disease, post-plastic surgery, post-prosthetic joint surgery, and post-dental implantation, which method comprises administering to said human an effective amount of a statin in hydrolyzed or unhydrolyzed form wherein said statin prepared as a pharmaceutical has been added to a liquid foodstuff.
4. The method of claim 3, wherein said statin is in hydrolyzed form.
5. The method of claim 3, wherein said statin is cerivastatin, lovastatin, mevastatin, simvastatin, fluvastatin, pravastatin, or NK-104.
6. The method of claim 3, wherein the liquid foodstuff is grapefruit juice.
7. The method of claim 3, wherein said liquid foodstuff is salad dressing or salad oil.
8. The method of claim 3, wherein said statin is emulsified in said foodstuff.
9. The method of claim 3, wherein said administering is on an intermittent protocol wherein the time period occupied by dosage administration is less than 50% of the time frame over which treatment is administered, as measured by the time between initial administration and assessment of results.
DISCLOSURE OF THE INVENTION
In one aspect, the invention is directed to a method to enhance bone growth in humans or other vertebrate animals by including in their diets foodstuffs or food supplements that contain effective amounts of bone growth enhancing compounds. These compounds can be the small molecule bone enhancing compounds of the formula Ar1—L—Ar2described hereinabove, bisphosphonates, or other bone growth enhancing agents such as BMPs. A particularly preferred group of compounds which can be administered in this was comprises the statins. Typical statins are of the formula:
wherein X in each of formulas (1), (2) and (3) represents a substituted or unsubstituted alkylene, alkenylene, or alkynylene linker of 2-6C optionally containing one heteroatom which is O, N or S;
Y represents one or more carbocyclic or heterocyclic rings; when two or more rings are present in Y, they may optionally be fused; and
R′ represents a cation, H or a substituted or unsubstituted alkyl group of 1-6C.
Thus, the invention is directed to methods to treat bone disorders by directly stimulating bone formation stimulating bone formation by including in the diet of a subject in need of such stimulation, a food or food supplement which contains a bone enhancing compound, especially a statin compound. Oral administration of the compounds of the invention, in particular the statins, enhances plasma concentration by avoiding hepatic first pass effects. Thus, their effects on bone and non-hepatic tissues are enhanced. In another aspect, the invention relates to administering the compounds that stimulate bone growth on an intermittent schedule in contrast to continuous administration.